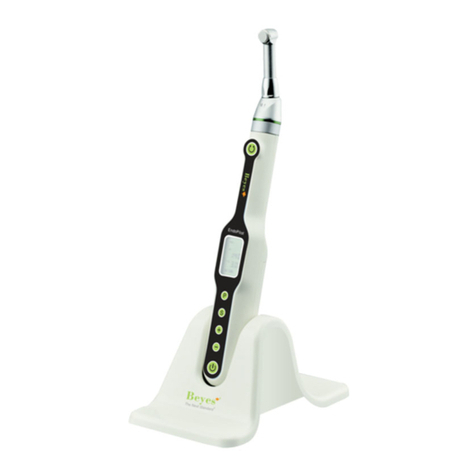
BEYES Canaview User manual

Canaview®
Intraoral Camera
Instruction for Use

2
Safety Issues
Check Canaview before Using It
Before each usage, check the outer surface of the Canaview for any signs of physical
damage or defect. The surface of the Canaview should have a smooth nish, with no
evidence of chipping or damage to either the handpiece housing or the lens section. To
help ensure proper hygiene and to protect against infectious disease, refer to "Section 4.1"
of this document and observe all device cleaning and patient protection recommendations
specied there.
Avoid Excessive Temperatures when Using Canaview
When in use, the LEDs in the Canaview may generate surface temperatures in excess of
106° F. (41° C). To avoid the potential risk of burn, do not use the Canaview in a single
hand-held position for a prolonged period.
Operate the Canaview as Directed
Always use the Canaview in accordance with the directions and recommendations
contained in this User Guide. Do not attempt to modify the Canaview or use it in system
congurations not specied in this document.
RF Interference Considerations
Although the Canaview equipment is designed to provide a reasonable degree of protection
from electromagnetic interference, according to IEC International regulations, it must be
installed at an adequate distance from electricity transformer rooms, static continuity units,
two-way amateur radios and cellular phones. To ensure proper operation, the latter can be
used only at a minimum distance of 5 feet (1.5m) from any part of the equipment.
Any instrumentation or equipment for professional use located near Canaview must
conform to Electromagnetic Compatibility regulations. Non-conforming equipment, with
known poor immunity to electromagnetic elds, may not operate properly unless they are
installed at a distance of at least 10 feet (3m) and supplied by a dedicated electric line.
Explanation of Symbols on the Canaview
Some of the symbols on the Canaview identify it as having met the requirements for sale
within the United States and for export internationally. The "CE" and "ETL" symbols are
examples of these types of marks.The remaining symbols provide either technical or
directive information.
Table of Contents
1. Introduction to Canaview..................................................................... 3
2. Software Setup.................................................................................... 4
3. Hardware Setup................................................................................... 4
4. Cleaning and Disinfecting.................................................................... 5
5 Warranty Statement.............................................................................. 7

3
Symbol Description
Indicates that the Canaview is Type BF equipment.
Indicates an attention to users to consult accompanying documents (this
User Guide) for more information on the Canaview.
Indicates that under certain ambient environmental conditions (especially
low humidity), the Canaview may be susceptible to electrostatic discharge
(ESD). Appropriate care and handling must be observed to avoid damage.
1. Introduction to Canaview
1.1. Package Contents
The Canaview system hardware consists of the following components.
• Camera
• Holder
• USB Cable
• Disposable Sheaths (50 pieces)
1.2. Computer System Requirements
Getting the best results from your Canaview begins with having a computer system
suitable for displaying and capturing video images. For optimum performance we
recommend:
• 2 Ghz Pentium 4 processor
• 1 GB of RAM minimum 2 GB or above recommended
• USB 2.0 interface with high power port
• CD-ROM or DVD drive
• Imaging Software
• Internet connectivity recommended
• Windows XP Professional or above with a minimum of Service Pack 2
IMPORTANT! USB bandwidth is shared among all USB devices. Achieving
optimum performance with the Canaview may not be possible if other USB
devices are in use at the same time.

4
1.3. Overview Illustrations
1
2
3
4
Camera lens and L.E.Ds
Capture
ON / OFF
Quick Connector
2. Software Setup
Scenario 1: If you already have imaging software, please contact your existing imaging
provider to assist you with the install.
Scenario 2: If you do not have imaging software, please refer to Canaview Quickvision
Users Guide.
3. Hardware Setup
3.1. Connecting the Canaview to computer
The USB cable used with the camera is a 2.5 meter (3 yards)
cable. The “ 5” plug connects to any available USB port
on the computer. The “ 6” plug connects to the camera
handpiece. It will take a few minutes for the computer to
recognize the camera.
3.2. Installing the Canaview Handpiece Holder
The Canaview Handpiece Holder turns off the camera when
the handpiece is inserted and turns on the camera when the
handpiece is removed. The holder is equipped with several
mounting options: (1) Can be wall-mounted with fastening
hardware, or (2) Can be attached to a wall or other acceptable
bonding surface with double-sided adhesive tape.
5
6

5
PLEASE NOTE: When selecting the mounting option for your Canaview, choose a
location that offers easy access during patient exams and safe storage afterwards.
In most practices, mounting the Canaview to the dental chair will provide the best
all-around solution.
3.2.1. Wall-Mounting Option (with Fasteners)
To install the handpiece by fastening it to a wall or other vertical surface, do the following:
1. Remove the handpiece from its holder before performing this procedure.
2. Remove the cutout block from the handpiece holder by loosening 2 at-head Phillips
screws.
3. Position the holder on a smooth stable vertical surface. Using the holes on the back of
the holder as guides, fasten the holder securely to the wall using 2 (#6 x 3/4) dry wall
screws (not supplied) or other hardware appropriate to the mounting surface.
3.2.2. Wall-Mounting Option (with Adhesive)
To install the handpiece by attaching it with adhesive tape to a wall or other vertical
surface, do the following:
1. Remove the handpiece from its holder before performing this procedure.
2. Remove the cutout block from the handpiece holder by loosening 2 at-head Phillips
screws.
3. Cut and trim a piece of double-sided adhesive tape (not supplied) to the size of the
back of the holder. Remove one side of the tape and attach it to the holder.
4. When the holder is correctly located, remove the other side of the adhesive tape and
fasten the holder securely to the wall or other surface.
4. Cleaning and Disinfecting Cameras
The Canaview intraoral cameras use disposable sheaths to ensure proper infection control.
Sheaths are intended for single-use only. Do not use the camera for intraoral use until a
new sheath has been properly tted over the handpiece. After each intra-oral use, remove
the sheath and dispose of it properly.
IMPORTANT! Be sure to disconnect the camera from the USB cable before
performing any cleaning procedures.
Step 1 Step 2 Step 3

6
4.1. Recommended Disinfectant
The following surface disinfectant has been found to be effective in achieving a desired
level of disinfection:
• Cavi-Wipes (Metrex Research, Kerr) or equivalent
4.2. Cleaning and Disinfecting the Camera Handpiece
In a clinical use environment, the health care provider should wear protective disposable
gloves and cover the camera with a hygienic barrier. Before using the camera the rst time,
and before every new patient, the following protocol is recommended:
1. Remove and discard all protective hygienic barriers and / or sheaths from the camera
prior to removing disposable gloves.
2. Place the camera on a tray covered by a disposable liner, or in a receptacle that can be
thoroughly disinfected.
3. Remove and discard gloves.
4. Wash hands and put on a new pair of disposable gloves.
5. Disconnect the camera from the USB cable.
6. If the camera or cable are visibly soiled (e.g., with blood or saliva), each should be
cleaned with a soapy cloth or paper towel, and then dried with a clean lint-free cloth or
paper towel.
7. Thoroughly wipe the camera and cable (if applicable) with the disinfecting product
recommended above and wait 30 seconds. Do not expose the camera cable connector
to any amount of liquid.
8. Repeat step 7. When the camera has been wiped two times, continue with the following
steps.
9. Remove potential chemical build-up from the camera by wiping it with a sterile lap
sponge saturated with de-ionized water.
10. Use a sterile dry lap sponge to dry the camera or cable, as needed.
11. Place the camera in a clean environment, ready for next use.
12. Reconnect the camera.
13. Remove and discard gloves.
4.3. Cleaning the Lens Surface
To clean the lens surface, moisten a soft cotton swab dipped in rubbing (isopropyl) alcohol.
Gently wipe the lens surface end-to-end in straight lines without applying pressure; do
not scrub. Allow the alcohol to evaporate from the lens surface. Repeat these steps, as
needed, until the lens surface is clean.

7
5. Beyes Limited Warranty Statement
SCOPE OF WARRANTY
BEYES Dental Canada Inc. ('BEYES') warrants to the original retail purchaser that it will
be at BEYES option to repair or replace components of the dental products manufactured
by BEYES (except for components not warranted under 'Exclusions') that are defective
in material or workmanship under normal use and service. BEYES’ obligation under this
limited warranty is limited to the repair or replacement of the applicable components.
This limited warranty shall only apply to defects that are reported to BEYES within the
applicable warranty period and which, upon examination by Beyes, prove to be defective.
This warranty extends only to the rst retail purchaser of a product and is not transferable
or assignable. Replacement components or products may be used and/or refurbished
components or products, provided they are of like quality and specications as new
components or products.
APPLICABLE WARRANTY PERIOD
The applicable warranty period, measured from the date of invoice to the original user, shall
be warranted for a period of 12 months
EXCLUSIONS
This limited warranty does not cover and BEYES shall not be liable for the following;
1. Defects, damage or other conditions caused, in whole or in part, by misuse, abuse,
negligence, alteration, accident, freight damage, negligent storage, tampering or failure
to seek and obtain repair or replacement in a timely manner;
2. Products which are not installed, used, and properly cleaned and maintained as
required or recommended in the BEYES 'Installation' and/or 'Installation/User’s
Manual' for the applicable product, including the specied structural and operational
environment conditions and electrical power requirements;
3. Products considered to be of a consumable or sterile nature;
4. Accessories or parts not manufactured by BEYES;
5. Charges by anyone for adjustments, repairs, replacement parts, installation or other
work performed upon or in connection with such products which are not expressly
authorized in writing in advance by BEYES;
6. Costs and expenses of routine maintenance and cleaning;
7. Representations and warranties made by any person or entity other than BEYES;

8. Matching of color, grain or texture except to commercially acceptable standards;
9. Changes in color caused by natural or articial light;
10. Custom manufactured products;
11. Alterations or modications to the product by any person or entity other than BEYES;
12. Products that would otherwise by covered under Sections 1 and 2 of this limited
warranty, but are acquired: (i) from a person or entity that is not BEYES or one of its
authorized dealers; or (ii) from a BEYES dealer that is not authorized to sell the product
at issue in the geographic territory where the purchaser is located, or is not authorized
to sell the product at issue within the medical, animal health or dental market, as the
case may be, in which the purchaser intends to use the product.
EXCLUSIVE REMEDY; CONSEQUENTIAL DAMAGES DISCLAIMER
Beyes’ obligation under this limited warranty is the repair or replacement of defective parts.
Beyes shall not be liable for and hereby disclaims any direct, special, indirect, incidental,
exemplary or consequential damages or delays, including, but not limited to, damages
for loss of prots or income, loss of use, downtime, cover and employee or independent
contractor wages, payments and benets.
WARRANTY DISCLAIMER
This limited warranty is Beyes only warranty and is in lieu of all other warranties, express
or implied. Beyes makes no implied warranties of any kind including any implied warranties
of merchantability or tness for a particular purpose. this warranty is limited to the repair or
replacement of defective parts.
Beyes Dental Canada Inc.
23-595 Middleeld Road
Toronto, Ontario, M1V 3S2
Canada
Tel: 1-855-603-1888
Fax: 1-855-720-1228
Email: [email protected]
Website: www.beyes.ca
MEDevice Union Ltd.
26 York Street
London, W1U 6PZ
United Kingdom
Tel: 44-20-3514-0949
Email: [email protected]
Website: www.beyes.ca
Printed in Canada
ENI013R2
Federal law restricts this device to sale by or on the order of a dentist, physician, or any other practitioner
licensed by the law of the states in which he or she practices to use or order the use of this device.
0197
Table of contents
Other BEYES Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Teeter
Teeter Neck Relax & Restore Duo Assembly & user instructions
Lowenstein Medical
Lowenstein Medical JOYCE SilkGel Instructions for use

chinesport
chinesport ARTIPRESS user manual

Bionics
Bionics PalmCare plus Operation manual

Riester
Riester ri-fox N quick start guide

otometrics
otometrics madsen itera ii user guide