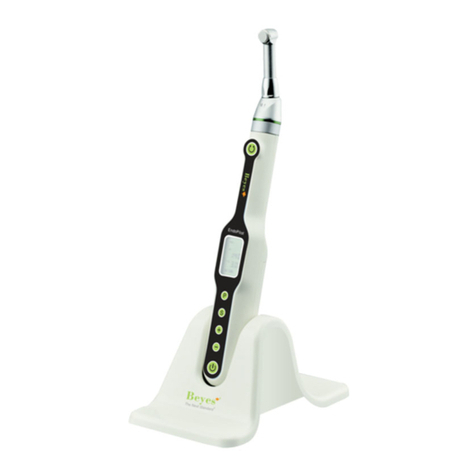
BEYES GPRO F1 User manual

Gutta Percha Obturation System
Instruction for Use

2
Thank you for purchasing GPro F1 Gutta-percha Obturation System developed by Beyes
Dental Canada Inc., Beyes has excellent Quality Control System. To guarantee correct and
safe operation, please read this Instruction Manual carefully before use. Depending on the level
of risk involved, safety requirements are classed under the following indications:
Danger: (Always refer to personal injury)
Warning: (Refer to possible damage to property)
Table of Contents
1. Introduction............................................................................................... 3
2. Installation ................................................................................................ 5
3. Operation.................................................................................................. 7
5. Technical Specication............................................................................. 9
6. Signs......................................................................................................... 10
7. Safety precautions.................................................................................... 11
8. Maintenance............................................................................................. 11
9. Troubleshooting........................................................................................ 12
10. After-sales service.................................................................................. 13
11. Environment protection........................................................................... 14
12. Beyes Limited Warranty Statement........................................................ 14

3
1. Introduction
1.1 Features
• Cordless and easy to use
• Fast and consistent gutta-percha ow
• Designed for both left handed or right handed individuals; both sides have display window and
operation button
• Variable preset temperature settings (150°C, 180°C, 200°C, 230°C)
• Auto shut off after 10 mins of idling
1.2 Intended use
A cordless backll obturation device that is designed to deliver a fast and continuous ow of
gutta-percha for precise root canal obturation.
1.3 Contraindications
Contraindications to the use of this device on patient includes
• Patient allergic to known natural latex and metals such as stainless steel, silver, copper
• Patient with hemophilia
• Patient or dentist with pacemaker implanted
• Patient with heart disease
Note: Use of device on pregnant women or children should be cautious.
1.4 Package includes
• GPro F1 gutta percha obturation gun
• Charging base/stand
• Power adapter
• Gutta-percha injection needles
Model Gauge Length
23G 24mm 23G 24mm
23G 28mm 23G 28mm
25G 24mm 25G 24mm
• Heat insulation hood
• Heat insulation sleeve of needle
• Pushing ram
• Cleaning brush
• Wrench
• Instruction Manual
• Qualied Certication
• Packing list

4
1. Gutta-percha injection needle
2. Heat insulation sleeve for injection needle
3. Heat insulation hood
4. Gutta-percha loading slot
5. Battery level
6. Temperature
7. On/off button
8. Pushing ram
9. Battery cover
10. Charging indicator
11. Charging base
12. Power adapter plug
13. Power adapter
14. Connecting hole for power supply
15. Trigger
16. Ram release
1.5 Diagram of Device
The Gpro F1 is equipped with a display screen and a control button on both the left and right
sides. The design of left and right sides are perfectly symmetrical, which enables either left-
hand or right-hand operation;
16

5
Light on the base will be yellow while battery is charging and will turn green once fully charged.
If the device is off the base, light ashes between yellow and green
Full battery charge takes approximately 2hr 30min
Notes: Charge battery for 3 hours before rst use.
Battery Indicator:
The battery indicator displays 5 grids with 1 as lowest to 5 as full battery level.
Indicator shows battery status while charging and it displays 5 grids when fully charged.
Battery should be charged when indicator shows 1 grid.
Warning:
Do not drain battery.
Always keep battery fully charged to maintain battery life.
Recharge battery if the device has not been used for more than 1 month.
2.2 Install needle
Warning:
Make sure the device is off
Allow 5 minutes for device to cool down
Screw in the gutta-percha injection needle to device
Note:
Needles of different sizes are included in the box
Use wrench in the package to fasten the needle.
Turn clockwise to tighten the needle
Note:
Turn counter-clockwise to untighten the needle
Figure 2 Figure 3 Figure 4
2. Installation
2.1 Charge device
Install the adapter plug for the power supply (Figure 2)
Plug in the power supply and connect it to the charging base (Figure 3)
Place the device on the base for charging. (Figure 4)

6
2.3 Adjust needle
Use wrench to bend needle to different angle for varies application.
Note:
Needles are made of silver which discolors after long-time exposed to air. It should be stored in
sealed environment.
Warning:
Needle remains hot after use. To remove the needle, turn off device and allow 5 minutes to cool
down.
Warning:
Before each use, clean, disinfect and sterilize the gutta-percha injection needle
Follow manufacturer instruction to disinfect the needle.
Do not autoclave this device.
Do not immerse this device into chemical disinfectant.
2.4 Load Gutta-percha
Select the right size of gutta-percha for operation
Insert gutta-percha into the loading slot
Warning:
Slot ts only 1 gutta-percha pellet.
Too many pellets can damage the device.
Use ram to insert from the back
Stop when you hear a click sound
2.5 Clip Heat insulation hood
The translucent insulation hood add an extra heat insulation. It protects doctor or patient from
touching the heating chamber. It protects the patient oral tissue and lip from scalding.
Warning:
Keep insulation hood on when device is in use.
Do not touch. Heating chamber is very hot and can burn.
Note:
Disinfect and sterilize the heat insulation hood before each use
Warning:
Make sure the device is off.
Allow 5 minute for device to cool down
Do not overtighten the screw.

7
3. Operation
3.1 Turn device on/off
Press and hold button for 3 sec. Device turns on. Display shows setting and status.
Press and hold button for 3 sec again. Device turns off. Display turns off.
Note: device automatically shut down after 10 minutes idling
3.2 Select Temperature
Press button to select the temperature setting.
Temperature setting cycles as shown below.
Gutta-percha injection needle Temperature
25G 180°C-230°C
23G 180°C-200°C
20G 180°C-200°C
The display shows the temperature of heating chamber.
1 second after the temperature is selected, heating chamber will heat up or cool down to the
target temperature.
3.3 Apply gutta-percha
Load gutta-precha pellet (see installation)
Make sure the temperature reaches the set temperature.
Squeeze and release the trigger. The ram then steps to push the gutta-percha through the
needle.
Warning:
Needle is hot and remains hot after use
Insulation hood should be in place to protect user and patients.
Note:
Needle and insulation hood should be cleaned and disinfected after each patient use.
3.4 Replace gutta-percha
Warning: Do not replace the gutta-percha when device is hot
1) Loaded gutta-percha pellet cannot be removed
2) Squeeze and release the trigger to push out the remaining gutta-percha
3) A click sound during release indicate the end of gutta-percha in the device
4) Push the release button to remove the ram from the back
5) Reload a gutta-percha
Warning: Do not replace the gutta-percha when device is hot

8
3.5 Clean heating chamber
After each use, remove all visible leftover gutta-percha obturator in the heat chamber with the
cleaning brush
1) Set the temperature to 200 °C and allow the device to heat up
2) Insert the cleaning brush into the back of the gun and push it through to the front
3) Draw the cleaning brush out from the front of the gun
Note: Do not remove the cleaning brush from the back
4) Repeat unit the chamber clean
4. Canal Obturation
1. Turn on device and insert a gutta-percha pellet into GPro F1 and adjust to the desired preset
temperature
2. Using GPro F1 activated tip, make contact with the upper part of lled master cone in the root
canal
3. Pull the trigger to extrude the melted gutta-percha obturator into the canal
4. Slowly retract the needle with continuous ow while withdrawing the tip from the canal
5. Follow with use of GPro P1 heating pen or vertical compaction device to compact obturation
material
Note: Fill from the bottom of the root canal to reduce or avoid the generation of bubbles.
Warning:
When the trigger is squeezed to ll the gutta-percha without retracting the needle, the injection
needle may break.
While the gutta-percha is still hot, use a warm vertical compaction device to compact obturation
material .
If there are bubbles in the root canal, use a small amount of material to ll the root canal for
many times. Use a little more material for each lling and use vertical compaction device to
press it down.
Note:
Turn off device after each lling
It stops gutta-percha from dripping out.

9
5. Technical Specication
5.1 Product specications
Sizes GPro F1 Gutta Percha Obturation Gun 31.9mm×152.5mm×114.9mm
Charging base 75.5mm×149.7mm×62.6mm
Weight GPro F1 Gutta Percha Obturation Gun 170g
Charging base 207g
Power adapter 167g
5.2 Technical parameters
Classication Class II(AC/DC power adapter)
Optional preset temperatures 150°C-180°C-200°C-230°C
Time consumption for charging About 2.5h (First charging needs 3 h)
Power supply Input AC100V-240V 50/60Hz 800mA
Output DC15V/1.6A
Battery capacity Chargeable battery 2000mAh
5.3 Environmental parameters
Working Storage
Temperature +5°C~+40°C -20°C~ +55°C
Humidity 30%~75% 10% ~ 93%
Air pressure 70kPa~106kPa 70kPa ~ 106kP
Warning:
Do not transport and store this device with hazardous articles such as combustible poisonous,
caustic, or explosive.

10
6 Signs
Product serial number Follow Instructions for Use
Manufacturer Date of manufacture
Type B applied part Class II equipment
Power switch Ordinary equipment
Used indoor only Device complies with WEEE
directive
Can be autoclaved Keep dry
Handle with care Recovery
Attention! Please refer to the accompanying documents.
Humidity limit for storage: 10% ~ 93%
Atmospheric pressure for storage: 70kPa ~ 106kPa
Temperature limit for storage: -20°C ~ +55°C
10 %
93 %
70kPa
106kPa
-20
55 max
min
IPXO
SN

11
7. Safety precautions
1. Do not use instruments other than the provided wrench to install, disassemble or pre-bent
gutta-percha injection needle.
2. Do not knock or scratch the the GPro F1 gutta percha obturation gun.
3. Do not place the the GPro F1 gutta percha obturation gun near an electronic device, phone,
radio or HD/ satellite TV as these may affect the temperature control of the GPro F1 gutta
percha obturation gun.
4. Keep heat carrier accessories such as the GPro F1 gutta percha obturation gun, gutta-
percha injection needle,heat insulation hood etc. under heating state away from inammable
and explosive materials.
5. Please keep the device clean before and after operation. Before each use, please clean,
disinfect and sterilize the accessories such as gutta-percha injection needle, heat insulation
hood and wrench.
6. Users should be equipped with adequate protection such as goggles, mask, etc. to prevent
cross-infection.
7. The product should be in strict accordance with relevant operation specications of medical
authority and relative regulations. The product can only be operated by trained doctors or
technicians.
8. Do not install, remove, or replace the heat insulation hood and injection needle under heating
state. If you need to replace the injection needle, please rst power off and wait for 5 minutes.
Five minutes later, if the the GPro F1 gutta percha obturation gun totally cools down, replace
the injection needle.
9. The injection needle must be correctly installed to prevent from falling off or gutta-percha
leakage during operation.
10. Do not use excessive force when pre-bending the injection needle to prevent the injection
needle from breaking. When the injection needle is bent or worn, the gutta-percha owing ability
may be deteriorated, and the operator should replace the new injection needle in time according
to the clinical condition;
11. Beyes is specialized in producing medical instrument. We are only responsible for the safety
on the following conditions:
a) The maintenance, repair, and modication are made by the manufacturer or the authorized
dealers.
b) The charged components are original of “Beyes” and operated according to instruction
manual.
8. Maintenance
8.1 Cleaning, disinfection and sterilization
After use, squeeze out all the residual materials inside heating chamber, power off the device,
pull the pushing ram out of the GPro F1 gutta percha obturation gun from the back side, and
remove the material on the top of pushing ram.
1) Cleaning of charging base and the GPro F1 gutta percha obturation gun
The charging base and the surface of the GPro F1 gutta percha obturation gun can be wiped
with a soft towel with a small amount of neutral detergent or disinfectant alcohol.
2) Heat insulation hood
Before rst use and before used to different patients, please clean, disinfect and sterilize it. It is
recommended to execute steam sterilization after washing with water or washing in ultrasonic
cleaner.
3) Gutta-percha injection needle
After being used on each patient, please change the needle each time. When there is a found
or suspected damage to the needle, place it in a xed recycling container.

12
4) Cleaning of heating chamber
When removing the residue inside the heating chamber and the loading slot, set the
temperature of the device to 200 °C, and then power off the device after squeezing all the
residual materials out. After pulling the pushing ram out from back part, insert the supplied
cleaning brush from the back of the device and pull it out from the front part of the GPro F1
gutta percha obturation gun. Please take care not to add any cleaning agent or chemicals to the
cleaning brush.
5) Pushing ram
Use sterile alcohol and gauze to remove any visible residue.
6) The gutta-percha injection needle, heat insulation hood, and wrench can be cleaned with
clean water as well as can be cleaned in an ultrasonic cleaner;
7) After each use, please sterilize the gutta-percha injection needle, heat insulation hood, and
wrench under high temperature and high pressure (134°C, 0.22MPa) for at least 4 minutes.
8) If the other accessories need to be cleaned or disinfected, please use gauze to pick up a
small amount of water or disinfectant to wipe the surface. Do not soak those accessories in the
cleaning solution.
9) Do not use volatile and difuent solvents for cleaning, which will damage the surface of the
device or cause the markings on the machine to fade.
8.2 Daily maintenance
When the device is not used, please turn off the power and unplug the power supply plug.
If the the GPro F1 gutta percha obturation gun is in a low battery state for a long time, the
service life of battery will be shortened. Please charge it in time if the battery level is low. When
the device is not used, please charge it for 1 hour once a month.
8.3 Repair of device
This product does not contain self-repairing spare parts. Repair should be carried out by a
designated professional or special repair shop.
9. Troubleshooting
Fault Cause Solution
After pressing
the “ON/OFF”
button, the
device is still
off.
1. Inadequate battery power
2. Battery is damaged.
3. The charging interface is short-
circuited, causing the lithium battery to
enter a protection state;
4. GPro F1 gutta percha obturation
gun is damaged.
1. Connect to power supply to
charge. / Replace the battery.
2. Replace the battery.
3. Remove the substance that
causes the short circuit, put the
device into the charging base to
charge, and then the device will
return to normal;
4. Contact local distributor or
manufacturer.
Gutta-percha
cannot ow
out from the
needle
1. The push ram has been pushed
to the end, indicating that the gutta-
percha has run out.
2. The pushing ram seal ring
is damaged.
3. The injection needle is damaged
and blocked
1. Pull back the pushing ram and
load a new gutta-percha stick.
2. Replace the pushing ram
3. Replace the injection needle
Automatic
shutdown
If there is no operation for 10 minutes,
the device will automatic powers off
Reboot

13
The pushing
ram cannot
be pulled out
The portion of the pushing ram that
enters the interior of the heating
chamber is xed by the cooling of the
gutta-percha.
1. Power on and set the
temperature to 200 °C. After the
temperature reaches the set value,
pull out the pushing ram back;
2. Contact your local dealer or our
company.
Charging
failure after
connecting to
power supply.
1. The power supply is not
correctly connected;
2. The power supply is
damaged, or the specication
doesn’t match.
3. There are impurities on the
contact thimble of charging
base.
1. Unplug and reconnect.
2. Replace the battery.
3. Wipe the thimble with alcohol,
dry it, and reconnect.
The service
time after
each
charging is
shortened.
The battery capacity becomes
smaller.
Send to the repair center.
ERROR code
appears
on display
screen
The heating chamber is damaged. 1. Send to the repair center.
2. Contact local distributor or
manufacturer.
If the problem still cannot be solved, please contact your local dealer or Beyes Dental Canada
Inc.
1. When the pushing ram is in the the GPro F1 gutta percha obturation gun, please do not
push or pull the pushing ram vigorously. When the gutta-percha is heated up to the preset
temperature, the pushing ram should be pushed by pulling the trigger for multiple times. If the
pushing ram does not move, try to push it manually with a slight force, and try to pull the trigger.
2. Please refer to the recommended temperature to set the preset temperature.
3. To remove all remaining material, please rst remove the injection needle, and then pull the
trigger to squeeze out all the residual material in the heating chambers. Be careful not to touch
the head of the the GPro F1 gutta percha obturation gun to avoid scalding. Power off, cool it
down slightly, and push the pushing ram down.
10. After-sales service
Starting from the date of sales, if the device does not function up to standard due to
manufacturer defect, our company will be responsible for the repair of device during its warranty
period. Please refer to the warranty card for warranty period and scope.

14
11. Environment protection
The device does not contain any harmful substances. It may be handled or disposed in
accordance with the relevant local regulations.
Note:
1) Without Beyes agreement and authorization, private modication of device may result in the
electromagnetic compatibility problem of that device or other devices.
2) The design and test of the GPro F1 gutta percha obturation gun complies with the related
operation regulations of electromagnetic compatibility.
12. Beyes Limited Warranty Statement
11.1 SCOPE OF WARRANTY
BEYES Dental Canada Inc. warrants to the original retail purchaser that it will be at BEYES
option to repair or replace components of the dental products manufactured by BEYES (except
for components not warranted under 'Exclusions') that are defective in material or workmanship
under normal use and service. BEYES’ obligation under this limited warranty is limited to the
repair or replacement of the applicable components. This limited warranty shall only apply to
defects that are reported to BEYES within the applicable warranty period and which, upon
examination by Beyes, prove to be defective. This warranty extends only to the rst retail
purchaser of a product and is not transferable or assignable. Replacement components or
products may be used and/or refurbished components or products, provided they are of like
quality and specications as new components or products.
11.2 APPLICABLE WARRANTY PERIOD
The applicable warranty period, measured from the date of invoice to the original user, shall be
as follows
GPro F1 are warranted for a period of 12 months
11.3 EXCLUSIONS
This limited warranty does not cover and BEYES shall not be liable for the following;
1. Defects, damage or other conditions caused, in whole or in part, by misuse, abuse,
negligence, alteration, accident, freight damage, negligent storage, tampering or failure to seek
and obtain repair or replacement in a timely manner;
2. Products which are not installed, used, and properly cleaned and maintained as required
or recommended in the BEYES 'Installation' and/or 'Installation/Operation Manual' for the
applicable product, including the specied structural and operational environment conditions
and electrical power requirements;
3. Products considered to be of a consumable or sterile nature;
4. Accessories or parts not manufactured by BEYES;
5. Charges by anyone for adjustments, repairs, replacement parts, installation or other work
performed upon or in connection with such products which are not expressly authorized in
writing in advance by BEYES;
6. Costs and expenses of routine maintenance and cleaning;
7. Representations and warranties made by any person or entity other than BEYES;
8. Matching of color, grain or texture except to commercially acceptable standards;
9. Changes in color caused by natural or articial light;
10. Custom manufactured products;
11. Alterations or modications to the product by any person or entity other than BEYES;
12. Products that would otherwise by covered under Sections 1 and 2 of this limited warranty,
but are acquired: (i) from a person or entity that is not BEYES or one of its authorized dealers;

15
or (ii) from a BEYES dealer that is not authorized to sell the product at issue in the geographic
territory where the purchaser is located, or is not authorized to sell the product at issue within
the medical, animal health or dental market, as the case may be, in which purchaser intends to
use the product.
11.4 EXCLUSIVE REMEDY; CONSEQUENTIAL DAMAGES DISCLAIMER
Beyes’ obligation under this limited warranty is the repair or replacement of defective parts.
Beyes shall not be liable for and hereby disclaims any direct, special, indirect, incidental,
exemplary or consequential damages or delays, including, but not limited to, damages for loss
of prots or income, loss of use, downtime, cover and employee or independent contractor
wages, payments and benets.
11.5 WARRANTY DISCLAIMER
This limited warranty is Beyes only warranty and is in lieu of all other warranties, express
or implied. Beyes makes no implied warranties of any kind including any implied warranties
of merchantability or tness for a particular purpose. This warranty is limited to the repair or
replacement of defective parts.
11.6 STATUE OF LIMITATIONS
No actions may be brought against Beyes for breach of this limited warranty, or implied
warranty, if any, or for any other claims arising out of or relating to the products, more than
ninety (90) days following expiration of the limited warranty period.

Beyes Dental Canada Inc.
23-595 Middleeld Road
Toronto, Ontario, M1V 3S2
Canada
Tel: 1-855-603-1888
Fax: 1-855-720-1228
Email: [email protected]
Website: www.beyes.ca
Lotus NL B.V.
Koningin Julianaplein 10, Ie Verd,
2595AA, The Hague,
Netherlands
Tel: +31645171879 (English)
+31626669008 (Dutch)
Email: [email protected]
Website: www.beyes.ca
Printed in Canada
ENI035
Rev.1/11.03.21
Federal law restricts this device to sale by or on the order of a dentist, physician, or any other practitioner licensed by
the law of the states in which he or she practices to use or order the use of this device.
0197
Table of contents
Other BEYES Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Ossur
Ossur UNITY Instructions for use

COOK Medical
COOK Medical Zenith Flex Series Instructions for use
AngioScore
AngioScore AngioSculpt RX Instructions for use

human care
human care Diana II Comfort 4ESB Service manual

Hellenbrand
Hellenbrand Hill-Rom Evolution LI156E Series user manual

AirSep
AirSep FreeStyle manual