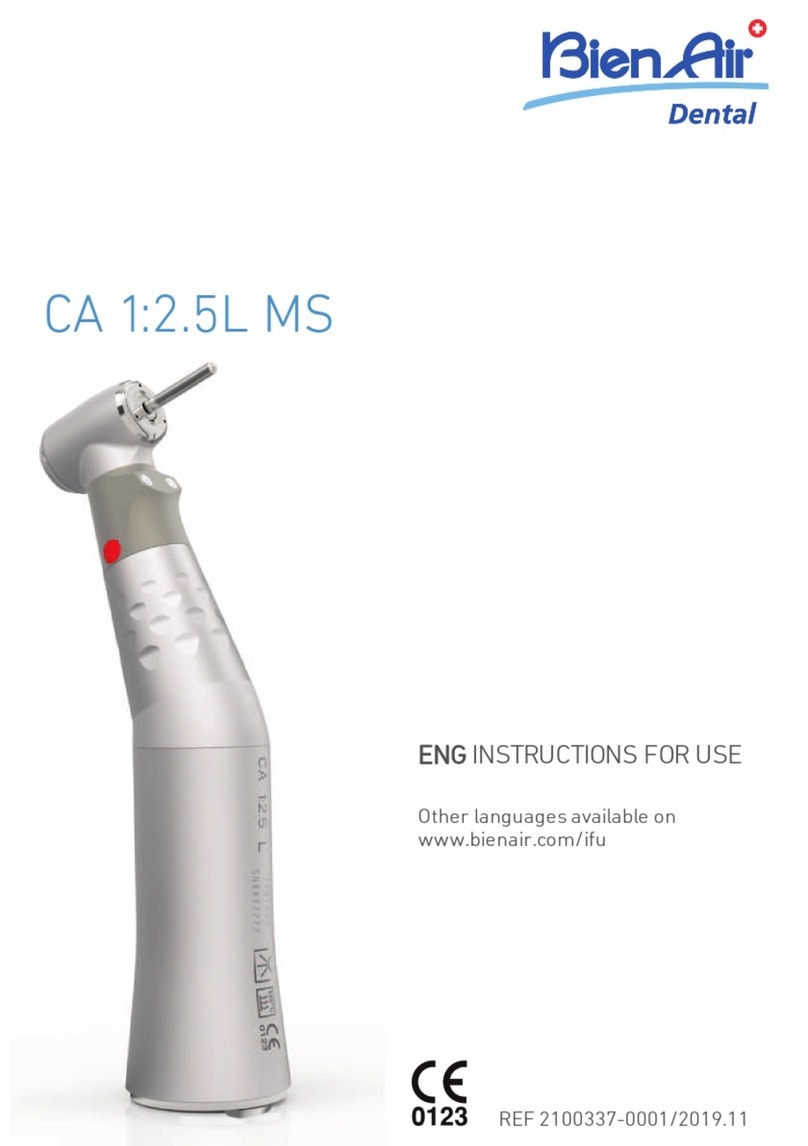
Bien Air CA ENDO User manual

REF 2100336-0000/2018.05
CA ENDO
ENG
INSTRUCTIONS FOR USE.
other languages available on
www.bienair.com/ifu

REF 2100336-0000/2018.05 CA ENDO • © Bien-Air Dental SA
Set supplied (REF)
1600955-001
Accessories (REF)
1600617-006 1600036-006 1600064-006 1600677-001 1600678-001

ENG
1
1 Symbols.......................................... 2
1.1 Description of symbols used ......2
2 Identification and intended use. 3
2.1 Identification ....................................3
2.2 Intended use ....................................3
3 Precautions for use ...................... 4
4 Description..................................... 5
4.1 Overview ...........................................5
4.2 Technical data .................................5
5 Assembly........................................ 6
5.1 Connection to the motor ...............6
5.2 Disconnection of motor ................6
5.3 Changing the file .............................6
6 Operation........................................ 7
7 Cleaning and servicing ................ 7
7.1 Maintenance ....................................7
7.1.1 Precautions for
maintenance ........................7
7.1.2 Suitable maintenance
products ................................7
7.2 Preliminary cleaning .....................8
7.3 Cleaning-disinfection ....................9
7.3.1 Manual cleaning/
disinfection ...........................9
7.3.2 Automatic cleaning-
disinfection ...........................9
7.4 Lubrication .................................... 10
7.4.1 Verifying cleanliness ...... 10
7.4.2 Lubrication ......................... 10
7.5 Sterilisation ................................... 10
7.6 Servicing ........................................ 10
8 Packing and disposal................. 11
8.1 Transport
and storage conditions ..............11
8.1.1 Packing ............................... 11
8.2 Disposal ..........................................11
9 General information................... 11
9.1 Terms of guarantee .................... 11
9.2 References .................................... 12
9.2.1 Set supplied (see cover) 12
9.2.2 Accessories (see cover) .12
Table of contents

2
ENG
Instructions for use
1Symbols
1.1 Description of symbols used
Symb Description Symb Description
Manufacturer. Serial number.
Separate collection of electric and
electronic equipment.
CAUTION! Consult accompanying
documents.
Provides an instruction that should
be observed for safety reasons.
Recyclable materials.
Refer to the accompanying
documents. (www.bienair.com/ifu.) Use rubber gloves.
Sterilisable in an autoclave up to the
specified temperature. Machine washable.
Movement in the direction
indicated.
After initial mechanical resistance,
fully tighten in the direction
indicated.
Movement to the stop in the direction
indicated. Back and forth movement.
Reference number.
Device supplied non-sterile.
SN
135°C
REF

ENG
3
2 Identification
and intended use
2.1 Identification
Type
Dental contra-angle (CA) with push-
button file locking.
Classification
Class IIa according to European
Directive 93/42/EEC relating to medical
devices. This medical device complies
with the legislation in force.
2.2 Intended use
Product intended for professional use
only.
The CA ENDO is intended for use in
endodontics by dentists and dental
professionals in a dental office.
CAUTION
Any use other than that for which this
device is intended is prohibited and
may prove dangerous.
CAUTION
The instructions contained in this
manual must be followed, in particular
the precautions for use.

4
3Precautions
for use
This medical device must be used by a
competent person, in particular
in
compliance with the legal provisions in
force regarding occupational safety, health
and accident prevention measures, and
these instructions for use.
In accordance with these provisions,
the user is responsible for ensuring he
or she only uses devices which are in
perfect working order.
In the event of irregular operation,
excessive vibrations, abnormal heating
or other signs suggesting that the
device is malfunctioning, work must be
suspended immediately.
In this case, contact a repair centre
approved by Bien-Air Dental SA.
CAUTION
Place the device on a suitable support
to prevent injury and infection.
CAUTION
Universal precautions, in particular
wearing of personal protective
equipment (gloves, a mask and safety
glasses), should be complied with by
medical personnel using or performing
maintenance on medical devices that
are contaminated or potentially
contaminated. Pointed and sharp
instruments should be handled with
great care.
CAUTION
Device is supplied non-sterile and must
be cleaned, disinfected, and sterilized
prior to first use and before each
subsequent use.
Clean, lubricate and sterilise the device
separately IMMEDIATELY after each patient.
CAUTION
Any excess maintenance product
(lubricant, cleaning and disinfection
products) present on the contra-angle
could find its way into the electric brush
motor and adversely affect its
operation.
Always observe the maintenance
instructions provided with each
product.
CAUTION
The CA ENDO is not designed for use in
an explosive atmosphere (anesthetic
gas).
CAUTION
Only use with a dental unit that
complies with the guidelines of
the EN 60601 standard.

ENG
5
FIG. 1
135°C
1
2
3
4Description
4.1 Overview
FIG. 1
(1) Transmission ratio marking strip
(2) Endodontic file (not provided)
(3) Push-button
4.2 Technical data
Note: (*) Indicative values. In the event
of the use of a longer or wider file, the
user is responsible for choosing the right
operational conditions that avoids all
risks to patients or third parties.
Follow the instructions for use, in
accordance with the files manufacturer’s
instructions.
Description Value
Standard coupling ISO standard 3964
Maximum operating
speed 9000 rpm
Maximal speed of motor
40000 rpm
Shanks for rotary and
oscillating
instruments
Type 1 according to
ISO 1797
Root-canal
instruments
According to ISO
3630-1
MAX motor speed
when tool at 300 rpm 1200 rpm (*)
MAX motor speed
when tool at 600 rpm 2400 rpm (*)

6
CA ENDO file
FIG. 2
• Shank diameter 2.35 mm, type 1
according to ISO 1797.
• Tool dimensions complying with
ISO 3630-1.
CAUTION
Follow the guidelines for use, according
to the tool manufacturer's instructions.
Never use a tool if the shank is not
conform.
5Assembly
5.1 Connection to the motor
CAUTION
Never insert a device on a rotating
micromotor.
1. Check that the device is completely
dry before connecting it to the motor.
2.
Install the CA on the motor connection
until ratcheting. For this, keep the motor
and the CA in the same axle.
3.
Apply light traction to the CA to check
that it is properly attached to the motor
connection.
4.
Carry out an operational test: switch
on the motor; start at low speed, then
gradually increase, outside of the
patient’s mouth.
5.2 Disconnection of motor
Remove the CA by pulling it away from
the motor, along its axle.
CAUTION
In the event of prolonged non use of the
device, do not leave it connected to the
motor. Risk of damaging the motor.
5.3 Changing the file
FIG. 3
Push-button tool locking.
1. Press the push-button and
simultaneously pull the file.
2.
Press the push-button, insert the
new file until locked in place and
release the push-button.
3.
Apply light traction to check that the
file is correctly locked.
CAUTION
Always check that the file is in place
and rotates freely. If it does not, contact
your usual supplier or Bien-Air Dental
SA for repair.
FIG. 2 FIG. 3
ø 2,35 mm
Type 1 / ISO 1797
1
23
3
2
1

ENG
7
6Operation
CAUTION
Insert a file before operating the device.
To prevent the push-button from
overheating, which could lead to burns,
it must not be pressed accidentally
when the device is rotating.
Soft tissue (e.g. tongue, cheek and lips,)
must be protected using a retractor or
dental mirror.
7 Cleaning and
servicing
7.1 Maintenance
7.1.1 Precautions for maintenance
• Before first use and IMMEDIATELY
after each procedure, clean, disinfect
and lubricate the device, then
sterilise it. Observing this procedure
eliminates any blood, saliva or saline
solution residues and prevents the
transmission system from being
blocked.
• Only instruments marked with the
logo can be cleaned in a washer-
disinfector machine.
• Do not immerse in an ultrasonic
cleaner.
• Only use original Bien-Air Dental
maintenance products and parts or
those recommended by Bien-Air
Dental SA. Using other products or
parts may cause faults during
operation and/or void the warranty.
File chuck mechanism (cutting tool)
Carry out cleaning – disinfection –
sterilisation without a file in the chuck
mechanism.
7.1.2 Suitable maintenance products
Automatic cleaning-disinfection:
• Low alkaline or enzymatic detergent
recommended for cleaning in a
washer-disinfector for dental or
surgical instruments (pH 6 - 9.5).
Manual cleaning/disinfection
•Spraynet.
• Detergent or detergent-disinfectant
(pH 6- 9.5) recommended for
cleaning-disinfection of dental or
surgical instruments. Surface-active
quaternary enzymatic/ammonium
detergent.
CAUTION
• Do not use detergents that are
corrosive or contain chlorine,
acetone, aldehydes or bleach.
• Do not submerge in physiological
liquid (NaCl).
• Check that both the steriliser and
water used are clean. After each
sterilisation cycle, remove the device
from the sterilisation unit
immediately to reduce the risk of
corrosion.

8
7.2 Preliminary cleaning
Preparation
FIG. 4
1. Disconnect the motor and remove
the file.
CAUTION
If there is a large amount of organic
contaminants, clean the exterior of the
device with disinfectant wipes.
FIG. 5
2.
Insert the Spraynet end-piece in CA
handle,
3.
Spray the exterior and interior of the
device for 1 second with Spraynet.
Carefully clean the surfaces using a soft
cloth. Disinfectant wipes may also be
used.
4.
Leave any liquid residue to drip-dry,
then wipe the exterior with a paper
towel or move onto the cleaning-
disinfection step immediately
(see 7.3, on page 9).
FIG. 4 FIG. 5
23
4

ENG
9
7.3 Cleaning-disinfection
7.3.1 Manual cleaning/disinfection
1. Dip the device in a tank containing
the appropriate detergent and, using a
soft brush, clean and disinfected, clean
the exterior of the device in accordance
with the instructions of the detergent
manufacturer (e.g. time, concentration,
temperature and renewal.).
2.
Rinse the interior and exterior of the
device with demineralised water
(< 38°C) for 30 seconds.
FIG. 6
3.
Spray the interior of the device with
Spraynet to remove the rinsing water
immediately, which will prevent damage
to or blockage of the internal
components, then dry the exterior
surfaces with paper towels.
7.3.2 Automatic cleaning-disinfection
CAUTION
Only for devices engraved with .
Washer-disinfector
Carry out automatic cleaning-
disinfection using an approved washer-
disinfector which complies with ISO
standard 15883-1 (e.g. Miele G 7781/G
7881 or Steris Hamo LM-25).
Detergent and washing cycle
Use a low alkaline or enzymatic
detergent recommended for cleaning in
a washer-disinfector for dental or
surgical instruments (pH 6 - 9.5) (e.g.:
neodisher® mediclean).
Select the washing cycle recommended
for the device and compatible with the
detergent manufacturer's indications
(e.g.: VARIO-TD)
CAUTION
Never cool devices by rinsing them.
FIG. 6
12
3

10
7.4 Lubrication
7.4.1 Verifying cleanliness
Visually inspect the device to ensure it
is clean. If necessary, clean again using
a soft brush.
7.4.2 Lubrication
Lubricate before each sterilisation or at
least twice a day. Only the Lubrifluid
spray must be used.
FIG. 7
1. Remove the bur from the device and
place the device in a cloth to remove
surplus lubricant.
2.
Select the correct end-piece and
remove the protective cap.
3.
Insert the end-piece of the can
Lubrifluid in the rear of the device's
handle.
4.
Actuate the spray for 1 second, and
clean the excess oil on the exterior.
7.5 Sterilisation
CAUTION
The quality of the sterilisation is highly
dependent on how clean the device is.
Only perfectly clean instruments may
be sterilised.
CAUTION
Do not use a sterilisation procedure
other than the one described below.
Procedure
Enclose the device and its accessories
in sterilisation bags which are large
enough to ensure the items can move
around, and which comply with the
standards in force (e.g.: EN 868-5).
Sterilise using steam on a class B cycle
as per EN 13060 / ISO 17665-1.
Note: All Bien-Air Dental SA contra-
angles are sterilisable in an autoclave up
to 135°C. Duration: 3 or 18 minutes,
depending on the national requirements
in force.
FIG. 8
After cleaning, disinfecting and
sterilising the device, and before using
it, start it up at moderate speed with a
file in the locking mechanism (FIG. 3,
step 2.) for 10 to 15 seconds to
distribute and remove the excess
lubricant.
7.6 Servicing
Never dismantle the device. For all
servicing or repair operations, you are
advised to contact your usual supplier
or Bien-Air Dental SA directly.
Note: Bien-Air Dental SA recommends
that the user has his or her dynamic
devices checked or serviced regularly.
FIG. 7 FIG. 8
1
2
34

ENG
11
8 Packing and
disposal
8.1 Transport and storage
conditions
Temperature between -40°C and 70°C
inclusive, relative humidity between
10% and 100%, atmospheric pressure
between 50 kPa and 106 kPa.
8.1.1 Packing
Pack the instrument in packaging
approved for steam sterilisation.
CAUTION
If not to be used for a prolonged period,
the device must be stored in a dry
environment. Clean, lubricate and
sterilise the instrument before reuse.
8.2 Disposal
The disposal and/or recycling of
materials must be performed in
accordance with the legislation in force.
The CA and its accessories must be
recycled. Electrical and electronic
equipment may contain dangerous
substances which constitute health and
environmental hazards.
Users should return devices to their
distributors or directly contact an
approved body responsible for
processing and recovering this type of
equipment
(European directive 2002/96/EC).
9 General
information
9.1 Terms of guarantee
Bien-Air Dental SA grants the user a
warranty covering any operating fault,
or material or manufacturing defect.
The warranty period is 12 months
from the date of invoicing.
In the event of a justified claim,
Bien-Air Dental SA or its authorised
representative will repair or replace the
product free of charge.
All other claims of any kind whatsoever,
particularly claims for damages or
interest, are excluded.
Bien-Air Dental SA cannot be held liable
for damage or injury and the
consequences thereof, resulting from:
• Excessive wear
•Infrequentuse
• Failure to observe the servicing,
assembly or maintenance
instructions
• Damage caused by unusual
chemical, electrical or electrolytic
influences
• Faulty air, water or electrical
connections.
CAUTION
The warranty becomes null and void if
damage and its consequences result
from incorrect servicing or modification
by third parties not authorised by Bien-
Air Dental SA.
Claims under the terms of the warranty
will be considered only on presentation,
together with the product, of the invoice
or the consignment note. The following
information must be clearly indicated:
The date of purchase, the product
reference and the serial number.

12
9.2 References
9.2.1 Set supplied (see cover)
9.2.2 Accessories (see cover)
REF Legend
1600955-001 CA ENDO
REF Legend
1600036-006 Spraynet, 500 ml cleaning
spray, box of 6
1600064-006 Lubrifluid, 500 ml spray
lubricant oil, box of 6

REF 2100336-0000/2018.05 CA ENDO • © Bien-Air Dental SA

REF 2100336-0000/2018.05 CA ENDO • © Bien-Air Dental SA
Bien-Air Dental SA
Länggasse 60
Case postale
2500 Bienne 6, Switzerland
Tel. +41 (0)32 344 64 64
Fax +41 (0)32 344 64 91
dental@bienair.com
Bien-Air Deutschland GmbH
Jechtinger Strasse 11
79111 Freiburg, Germany
Tel. +49 (0)761 45 57 40
Fax +49 (0)761 47 47 28
ba-d@bienair.com
Bien-Air España, SAU
Entença, 169 Bajos
08029 Barcelona, Spain
Tel. +34 934 25 30 40
Fax +34 934 23 98 60
ba-e@bienair.com
Bien-Air USA, Inc.
5 Corporate Park
Suite 160
Irvine, CA 92606 USA
Phone +1 800-433-2436
Phone +1 949-477-6050
Fax +1 949-477-6051
dental@bienair.com
Bien-Air France Sàrl
19-21, rue du 8 Mai 1945
CS 30310
94113 Arcueil, France
Tel. +33 (0)1 49 08 02 60
Fax +33 (0)1 46 64 86 58
ba-f@bienair.com
Bien-Air Italia S.r.l.
Via Vaina 3
20122 Milano, Italy
Tel. +39 (02) 58 32 12 51
Fax +39 (02) 58 32 12 53
ba-i@bienair.com
Bien-Air UK Ltd
Arundel House,
Unit 1 - Ground Floor
Amberley Court,
Whitworth Road
Crawley, RH11 7XL, England
Tel. +44 (0)1293 550 200
Fax +44 (0)1293 520 481
ba-uk@bienair.com
Bien-Air Asia Ltd.
Nishi-Ikebukuro
Daiichi-Seimei Bldg. 10F
2-40-12 Ikebukuro, Toshimaku
Tokyo, 171-0014, Japan
Tel. +81 (3) 5954-7661
Fax +81 (3) 5954-7660
ba-asia@bienair.com
Beijing Bien-Air
Medical Instrument
Technology Service Co. Ltd.
Room1415,
Block B Lucky Tower,
No 3 Dongsanhuan Beilu,
Chaoyang District,
Beijing 100027, China
Tel. +86 10 6567 0651
Fax +86 10 6567 8047
ba-beijing@bienair.com
www.bienair.com
▦℻ゑ㦬棂◉₫ₘ
▦℻ゑ㦬棂◉₫ₘ
䘾▦恾⚆ㄇ䰞⮶
䘾▦恾⚆ㄇ䰞⮶☵
%ㄶ⸳
%ㄶ⸳
Table of contents
Other Bien Air Medical Equipment manuals
Popular Medical Equipment manuals by other brands
BHM Medical
BHM Medical MINISTAND 865 Series Technical manual

Creative Medical
Creative Medical PC-60F user manual

NRS Healthcare
NRS Healthcare P64090 User instructions

burmeier
burmeier Dali instruction manual
Seers Medical
Seers Medical CLINNOVA Gynae Pro Instructions for use
BD
BD EnCor Enspire Quick reference guide