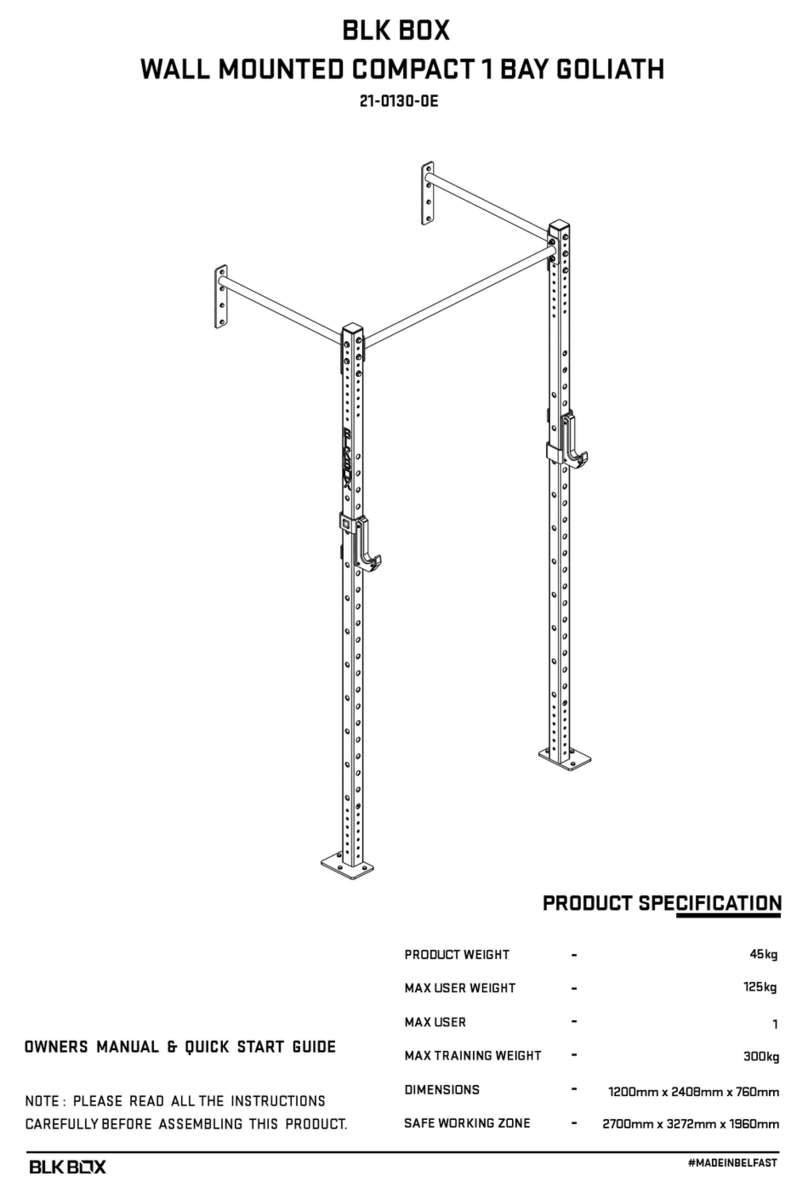
biodex 950-440 Installation and operating instructions

BALANCE SYSTEM SD (with v4.X software)
OPERATION/SERVICE MANUAL
950-440
950-441
950-444
FN: 17-100 Rev B 5/17

2 Biodex Medical Systems, Inc. © 2017
BALANCE SYSTEM SD (with v4.X software)
This manual covers installation and operation procedures for the following products:
950-440 System, Balance SD, 115 VAC
950-441 System, Balance SD, 230 VAC
950-444 System, Balance SD, 100 VAC

Balance System SD (with v4.X software) 3
Table of Contents
Definition of Symbols................................................................................................................ 6!
Product Certifications and Classifications.................................................................................. 7!
Before Proceeding ..................................................................................................................... 8!
Important Safety Information .................................................................................................... 8!
Biodex Warranty...................................................................................................................... 10!
1.!Introduction ...................................................................................................................... 13!
2.!Assembly and Installation.................................................................................................. 14!
Printer Installation ....................................................................................................... 14!
Parts and Adjustments................................................................................................. 14!
Power-up ..................................................................................................................... 15!
Power-down................................................................................................................. 15!
Connecting Components ............................................................................................. 16!
3.!Clinical Considerations...................................................................................................... 18!
General Clinical Considerations ................................................................................... 18!
4.!Applications of Body Weight Support Devices .................................................................... 19!
The Biodex NxStep Unweighing System........................................................................ 19!
The Biodex FreeStep SAS ............................................................................................. 20!
5.!Getting Started.................................................................................................................. 21!
Patient Setup Information Screen ................................................................................. 22!
Patient Setup Information Screen Parameters ............................................................... 23!
The Select Patient Screen ............................................................................................. 24!
Patient Setup Screen with optional Parameters............................................................. 25!
The Additional Information Screen............................................................................... 26!
The Diagnostic Information Screen .............................................................................. 27!
The G-Code Calculator Options Screen......................................................................... 27!
6.!Training Modes ................................................................................................................. 28!
Percent Weight Bearing Training .................................................................................. 28!
Weight Shift Training ................................................................................................... 31!
Motor Control Training ................................................................................................ 35!
Maze Control Training ................................................................................................. 37!
Random Control Training............................................................................................. 39!
7.!Testing Modes................................................................................................................... 42!
Sensory Integration Tests ............................................................................................ 43!
Clinical Test of Sensory Integration and Balance – CTSIB or m-CTSIB (Modified CTSIB) .. 44!
BESS Test..................................................................................................................... 48!

4 Biodex Medical Systems, Inc. © 2017
Limits of Stability (LOS) Test ........................................................................................ 50!
Postural Stability Test .................................................................................................. 53!
Motor Control Test ...................................................................................................... 56!
Bilateral Comparison Test............................................................................................ 59!
Fall Risk Test ............................................................................................................... 62!
8.!System Utilities.................................................................................................................. 65!
Reports........................................................................................................................ 65!
View Test Results and Print Report............................................................................... 66!
View and Print Progress Report .................................................................................... 75!
Configuration .............................................................................................................. 79!
System Configuration .................................................................................................. 79!
Balance System SD Configuration................................................................................. 81!
Create, Save, and Recall Custom Protocols................................................................... 84!
Create, Save, and Recall Custom Sensory Tests............................................................ 86!
Deleting Patient Files ................................................................................................... 90!
Printing and Editing Stored Results .............................................................................. 91!
Exporting Patient Data ................................................................................................. 92!
Importing Patient Data................................................................................................. 93!
System Maintenance .................................................................................................... 94!
Restore from USB......................................................................................................... 96!
9.!Software Updates .............................................................................................................. 98!
10.!Clinical Codes and Normative Data.................................................................................... 99!
ICD-10 (ICD-10-CM) ..................................................................................................... 99!
G-Codes..................................................................................................................... 100!
Sample Reports with G-Code – Percent Weight Bearing Training ................................. 104!
Sample Reports with G-Code – CTSIB/m-CTSIB Test.................................................... 105!
Normative Data.......................................................................................................... 109!
Fall Risk Test Normative Data .................................................................................... 110!
11.!System Specifications ...................................................................................................... 113!
12.!Maintenance.................................................................................................................... 114!
Cleaning Instructions................................................................................................. 114!
General Maintenance Procedures ............................................................................... 114!
13.!Electromagnetic Compatibility ......................................................................................... 115!
Conformance to Standards ........................................................................................ 115!
Accompanying EMC Documents................................................................................. 115!
List of Cable Accessories ........................................................................................... 115!
Declaration of Conformity.......................................................................................... 116!
Recommended Separation Distances.......................................................................... 118!

Balance System SD (with v4.X software) 5
Operating Temperature ............................................................................................. 118!
14.!Parts and Assembly Illustrations ...................................................................................... 119!
Appendix A: Data Definitions and Interpretation ................................................................... 125!
Appendix B: Interpretation of Reports ................................................................................... 128!
Appendix C: CSV file export (Balance SD and BioSway) .......................................................... 134!
Appendix D: Log Transformation .......................................................................................... 154!

6 Biodex Medical Systems, Inc. © 2017
Definition of Symbols
The following symbols and their associated definitions are used and implied throughout this
manual.
Symbol Definition
Carefully read these instructions prior to use
Caution
General Warning
General Mandatory Action
Dangerous Voltage
“On” Power
“Off” Power
Earth (ground)
Alternating Current
Fuse
USB Connector/Cable
Waste in Electrical Equipment
Date of Manufacture
Manufactured By
Type B Applied Part
CE Mark
CE Mark for products with EC Certificate
Certified for Safety by ETL Intertek
Balance System SD (with v4.X software) 7
Product Certifications and Classifications
The Balance System SD has received the following certifications, and falls within the
following classifications:
!ETL Listed Electrical Equipment, Laboratory Use; Part 1, General Requirements for Safety
conforms to UL 60601-1, CAN/CSA C22.2 No: 601-1-M90, IEC 60601-1, IEC 60601-1-4 and
IEC 60601-1-2 and CE Marked.
!FDA Class II Equipment.
!EC Certificate: EC # 4132458.
NOTE: Circuit diagrams for this product are provided in the Schematics section at the
back of this manual.
!Type B Applied Part.
!Electromagnetic Compatibility: This equipment complies with the Medical Equipment ICC
60601-2 EMC Standard.
Authorized European Community Representative:
Emergo Europe
Prinsessegracht 20
2514 AP, The Hague
The Netherlands

8 Biodex Medical Systems, Inc. © 2017
Before Proceeding
NOTE: The warnings, cautions and instructions provided in this manual must be
read, followed and kept available for consultation at all times. Observing the
information, instructions, and procedures presented throughout this manual is
essential for using this product both properly and safely.
SPECIFIC CAUTIONS
●Allow only qualified, trained personnel to operate or service this product.
●If the equipment is used in a manner other than specified in this operation
manual, the protection provided by the equipment may be impaired and
results could be compromised.
●Never leave patient unattended.
EN GARDE SPÉCIFIQUES
●Permettez au personnel seulement autorisé, entraîné de faire marcher ou
assurer l'entretien de ce produit.
●Si l'équipement est utilisé dans une manière autre qu'indiqué dans ce
manuel d'opération, la protection fournie par l'équipement peut être
diminuée et les résultats pourraient être compromis.
●Ne quittent Jamais le patient sans surveillance.
CAUTION: Unauthorized modifications to this product are not permitted and will
void the manufacturer’s warranty. Unauthorized modification of the product may
result in a hazard to the user and/or patient. Do not modify this equipment
without authorization from the manufacturer.
ATTENTION: Les modifications faites sans autorisation à ce produit ne sont pas
permises et va faire le vide la garantie du fabricant. La modification faite sans
autorisation du produit peut s'ensuivre dans un hasard à l'utilisateur et-ou le
patient. Ne modifiez pas cet équipement sans autorisation du fabricant.
Important Safety Information
CAUTION: Federal Law restricts this device to sale by, or on the order of a
medical practitioner. When prescribed for therapeutic purpose, a physician
should clearly define the parameters of use (i.e., total work, maximum heart
rate, etc.) to reduce the risk of patient injury.
ATTENTION: La Loi Fédérale restreint cet artifice à la vente par, ou sur
l'ordre d'un praticien médical. Quand prescrit pour le but thérapeutique, un
docteur devrait clairement définir les paramètres d'utilisation (c'est-à-dire,
travail total, taux maximum du cœur, etc.) pour réduire le risque de
blessure patiente.
Follow the assembly and installation instructions document.
Before using this device, read the entire operation manual carefully. Failure
to read the manual may result in user error or inaccurate data. Be sure to
save all provided documents for future reference.

Balance System SD (with v4.X software) 9
Make certain to understand all warning and caution labels as explained in
the Before Proceeding section of this manual.
This product should be used only as specified in the operation manual.
The Balance System SD is designed for use in a patient environment.
See Chapter 10 for Balance System SD specifications.
For product specifications, refer to the Table of Contents.
This medical electrical equipment requires special precautions regarding
EMC and must be installed and placed into service according to EMC
information provided in this manual. Electromagnetic compliance definition
is provided in the Chapter 13.
Reference Cleaning and Maintenance instructions in Chapter 12.
CAUTION: Operation for: 115-230 VAC, 50/60 Hz.
ATTENTION: Opération pour 115-230 VAC, 50/60 Hz.
WARNING: Only use approved power supplies.
AVERTISSEMENT: N'utiliser que les alimentations homologuées
CAUTION: To avoid risk of electric shock, this equipment must only be
connected to supply mains with protective earth.
ATTENTION: Pour éviter le risque de choc électrique, cet équipement doit
uniquement être connecté à un approvisionnement conduites avec la terre
protectrice.
CAUTION: The plug is considered the method of disconnecting the product
from main power. Do not place the product in a position where the plug is
not easily accessible.
ATTENTION: Le bouchon est considérée comme la méthode de déconnexion
du produit d'alimentation. Ne placez pas le produit dans une position où le
bouchon n'est pas facilement accessible.

10 Biodex Medical Systems, Inc. © 2017
CAUTION: The product is intended to remain in one location during
operation. The product is provided with wheels for relocation, and should
be used when performing this operation. One person can move the product.
ATTENTION: Le produit est voulu rester dans un emplacement pendant
l'opération. Le produit est fourni avec les roues pour la relocalisation, et
devrait être utilisé en exécutant cette opération. Une personne peut
déplacer le produit.
Biodex Warranty
Instrumentation
A. This equipment and its accessories are warranted by BIODEX MEDICAL SYSTEMS,
INC., against defects in materials and workmanship for a period of two years (2
years for parts, 1 year for labor) from the date of shipment from BIODEX MEDICAL
SYSTEMS, INC. During the warranty period, BIODEX MEDICAL SYSTEMS, INC. will in its
sole discretion, repair, recalibrate or replace the equipment found to have such
defect, at no charge to the customer.
EXCEPT AS STATED ABOVE, THERE ARE NO WARRANTIES, EXPRESSED OR IMPLIED,
INCLUDING WITHOUT LIMITATION WARRANTIES OR MERCHANTABILITY OR FITNESS FOR
USE. BIODEX DOES NOT ASSUME LIABILITY FOR INCIDENTAL, CONSEQUENTIAL OR
INDIRECT DAMAGES INCLUDING LOSS OF USE, SALES, PROFITS OR BUSINESS
INTERRUPTION.
B. This warranty does not apply if the product, as determined by BIODEX MEDICAL
SYSTEMS, INC., is defective due to abuse, misuse, modification or service performed
by other than a BIODEX MEDICAL SYSTEMS, INC. authorized repair and calibration
facility. Misuse and abuse include, but are not limited to, subjecting limits and
allowing the equipment to become contaminated by radioactive materials.
C. In order to obtain warranty repair service, the equipment or system component
must be returned freight pre-paid to one of our facilities. The Return Materials
Authorization number (R.M.A. #) should be included, along with a statement of the
problem. Equipment or system component will be returned transportation prepaid.
Calibration
A. Instruments are warranted to be within their specified accuracy at the time of
shipment. If a question arises and BIODEX MEDICAL SYSTEMS, INC. determines that the
initial calibration is in error, the instrument will be recalibrated at no charge.
B. Mechanical products are warranted to meet written specifications and tolerances at
the time of shipment.
C. The return policy is as stated in paragraph 1.C.

Balance System SD (with v4.X software) 11
Warranty is Non Transferable.
Non-Warranty Service
A. Repairs and/or replacements not covered by this warranty may be performed by
BIODEX MEDICAL SYSTEMS, INC. at a factory authorized service location. Estimates of
repair charges may be requested, however, a charge for estimate preparation may
apply if the repair is later not authorized by the customer.
B. The cost of transportation into and out of the service location will be the responsibility
of the customer.
Service Procedure
A. If a service problem exists, take the following action:
1. Check to see that the problem occurs more than once.
2. Refer to the instruction manual and operations procedure.
3. Refer to the instruction manual Troubleshooting Guide.
B. If a service problem still exists:
1. Call BIODEX MEDICAL SYSTEMS, INC., Service Department at (800) 224-6339.
2. Keep yourself and the phone next to the equipment.
3. Service will ask for a brief description of the problem. We will ask specific questions
about the malfunction that occurred. This diagnostic process may take a few
minutes; therefore, call us when you can set aside an uninterrupted block of time.
4. After taking the information, we will advise on the action we will take.
5. Sometimes service personnel must consult with engineering and it may take time to
get back to you. Be sure to let the service representative know your schedule in
order for a call back to be made at a convenient time.
6. The return call may be from a person other than the one to whom you first reported
the problem.
7. After analyzing the problem, we will decide if the unit can be repaired on site, or
replacement parts will be sent.
8. If the unit must be returned, Biodex will provide a return materials authorization
number (R.M.A. #.) Pack the table in the carton that it was originally shipped in. It is
the customer's responsibility for any damage that occurs during shipping.
9. Non-warranty/non-service contract charges for repair are as follows:
a. Materials.
+
b. Time.
+
c. Travel Zone.
12 Biodex Medical Systems, Inc. © 2017
Contact information
Manufactured by:
Biodex Medical Systems, Inc.
20 Ramsey Road, Shirley, New York, 11967-4704
Tel: 800-224-6339 (Int’l 631-924-9000)
Fax: 631-924-8355
email: supportservices@biodex.com
www.biodex.com

Balance System SD (with v4.X software) 13
1. Introduction
Intended Use
Featuring seven test protocols, six training modes and intuitive touch-screen operation, the
Balance System SD allows testing and training in both static and dynamic formats. Extremely
versatile, it is the only system that provides a balance assessment tool for concussion
management plus closed-chain, weight-bearing assessment, and training for lower extremity
patients.
Indications for Use
Using this unique device, clinicians can assess neuromuscular control by quantifying the
ability to maintain dynamic bilateral and unilateral postural stability on a static or unstable
surface.
Use any of seven test protocols including Limits of Stability, Postural Stability and Clinical Test
of Sensory Integration of Balance (CTSIB). The Balance System SD serves as a valuable training
device to enhance kinesthetic abilities that may provide some degree of compensation for
impaired proprioceptive reflex mechanisms following injury.
An easy-to-follow touch-screen format makes the system simple to learn and operate, leading
the user step-by-step through testing protocols and training modes. All test results and training
sessions are documented on easy to read 8.5" x 11" reports that can be placed into a patient’s
file. Comparisons to normative data can be made for population-specific tests.

14 Biodex Medical Systems, Inc. © 2017
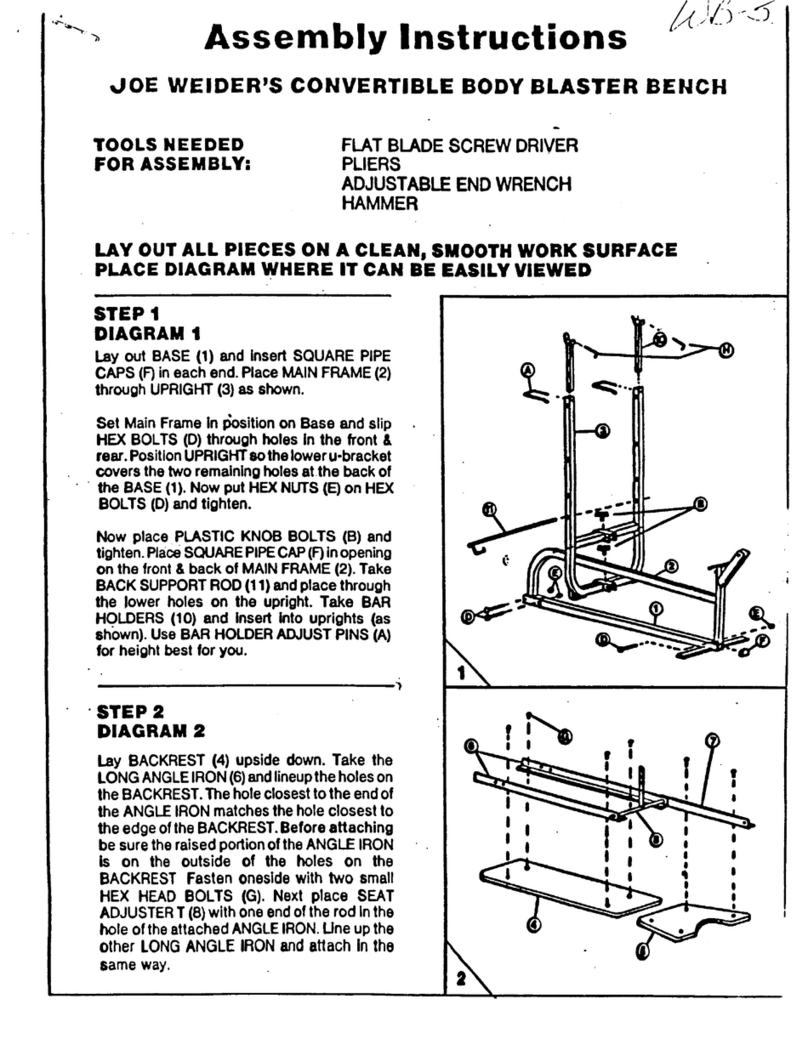
2. Assembly and Installation
The Balance System SD is shipped in a single carton. Except for the printer, which the user
must install as explained below, the entire system is factory assembled and ready to operate.
Printer Installation
1. Refer to the supplied printer manual to unpack the printer and ensure that it has not been
damaged by shipping.
2. Position the printer on the Biodex-provided printer stand.
3. Locate the printer power cable. Plug the small end into the power receptacle on the back of
the printer.
4. Insert the AC plug end of the printer power cable first into the Biodex-provided power
adapter, and insert the adapter plug into the power cable receptacle on the back, lower
base of the Balance System. Do not connect any other equipment to this receptacle.
5. Locate the Biodex-provided USB cable that will be pre-installed in one of the USB ports on
the Balance System display. Connect the other end of the cable to the port at the back of
the printer.
6. Ensure both cables are positioned such that they will not interfere with the patient or get
caught in the Balance System platform or handles.
7. Refer to the printer manual for directions on installing ink cartridges and paper.
8. With power ON to the Balance System, press the <Power ON> switch on the printer. Refer to
the printer manual for additional printer information.
Figure 2.1. Connect the power cable and USB cable to the rear of the printer.
Parts and Adjustments
Mechanical adjustments to the Biodex Balance System are straightforward and
uncomplicated. In fact, there are only three adjustments that need be addressed to

Balance System SD (with v4.X software) 15
accommodate any patient: Support Handle Position, Display Height, and Display Tilt. All other
test and exercise functions are software controlled.
To Adjust the Support Handle:
1. To position the Support Handle for patient use, hold the Support Handle while pulling out
on the Support Handle Release Pin. Rotate the handle to the desired position. Release the
pin to lock the Support Handle in place.
2. To release the Support Handle ensuring that it cannot be used by the patient, hold the
handle while pulling out on the Support Handle Release Pin. Fully lower the handle and
release the pin.
To Adjust the Display Height:
1. Loosen the Display Height Locking Knob.
2. Pull up or push down on the display until the desired height is achieved.
3. Tighten the locking knob to secure the display in the desired position.
NOTE: Position the display enabling the patient to look straight at it. This will help ensure
good posture during the test or exercise session.
To Adjust the Display Tilt:
Tilt the Display as required by patient or testing/exercise protocol.
Power-up
When the unit is plugged in, the display will automatically power up.
Power-down
In order to prevent the device’s database from becoming corrupted, it is essential that the
correct power-down sequence is performed. Always turn off the display, by touching the X in
the upper right corner of the home screen, followed by Shut Down.
Figure 2.2. Power-down sequence.
1
2

16 Biodex Medical Systems, Inc. © 2017
Note: If the display is shut down, but not unplugged, it can be turned back on by pressing the
rocker button on the bottom of the display.
Figure 2.3. Use the rocker button on bottom of the monitor to power the device back on.
Once the display has finished its shut down sequence, power may be removed from the system.
CAUTION: Do not unplug the device before powering down the display!
ATTENTION:
Ne débranchez pas l 'appareil avant de mettre l' écran hors tension!
Connecting Components
In addition to the printer that is shipped with the Balance System SD, other printers may also be
used with the device. Most Windows 7 printers should be compatible with the Balance System
Figure 2.4 Do not unplug the device before powering down the
display.

Balance System SD (with v4.X software) 17
display, but the drivers for various printers may need to be installed. For help with this, please
call Biodex Customer Support at 631-924-9000, Option 3. Similarly, any Windows 7 keyboard or
mouse will also automatically connect using one of the USB connections.
It is possible to connect the device to a printer wirelessly. Please call Customer Support for
instructions.
An external monitor can also be connected via the VGA port on the bottom of the display. Once
the external monitor’s cable is connected, the <Mirror to External Monitor> button in System
Utilities must be selected. (This button is accessed by the following navigation steps from the
Home screen: Utilities > Configuration > System Configuration > Screen Configuration.)

18 Biodex Medical Systems, Inc. © 2017
3. Clinical Considerations
Prior to allowing any patient to use this device, make certain to read and comprehend this
entire manual. Ensure that you are completely familiar with all aspects of adjustment, training
and testing, as well as patient history. Be sure to adhere to the following clinical guidelines at
all times when using this system.
NOTE: Never allow a patient to use the Balance System while unsupervised.
General Clinical Considerations
All users should have a verbal understanding of the Balance System prior to stepping on the
device.
To ensure patient safety, begin each session with the balance platform in the ‘locked’ or static
position.
NOTE: The Balance System automatically places the platform in the locked position when the
unit is turned ON, or after a time period of three minutes when the system is not in use.
Adjust support rail and biofeedback display for patient comfort and safety.
When dealing with post-operative patients, ensure they possess adequate muscular control to
stabilize the joint prior to placing them on the foot platform. Inadequate muscular control could
lead to increased joint translation.
When patients are working with their eyes closed, ensure that a clinician is ready to assist in
case of loss of balance.
Since the entire lower extremity is required to work to maintain good balance, ensure that
supporting structures above and below the joint are adequately strengthened prior to beginning
rehabilitation on this device.
For optimal operation, ensure the patient is standing in the center of the platform.
Patients should progress from ‘hands-on’ to ‘hands-off’ the support handle. This will ensure
that new or unstable patients have an adequate understanding of the Balance System and will
help protect the patient against sudden or unexpected movement of the platform.
Position the display enabling the patient to look straight at it. This will help ensure good
posture during the test or exercise session.
There is a patient learning curve that must be considered when testing with this device. Clinical
research suggests three trials be performed prior to testing. For dynamic balance testing, the
default settings are preselected with three trials per side. This should assist with the patient’s
familiarization with the device and result in better data averaging.

Balance System SD (with v4.X software) 19
4. Applications of Body Weight Support
Devices
The Biodex NxStep Unweighing System
Figure 4.1. The NxStep ready for use with the Biodex Balance System SD.
The Biodex NxStep Unweighing System is ideal for use with the
Biodex Balance System SD.
The loss of the ability to ambulate can be one of the most debilitating aspects of many
neurological and musculoskeletal disorders. Any of the three main components of locomotion –
posture, balance and coordination – can be affected by a variety of neurological or
musculoskeletal pathologies resulting in the disruption of an individual’s ability to walk
normally.
Partial Weight Bearing (PWB) gait therapy has shown great promise in helping a wide variety of
impaired patients as they relearn the walking function. It is an appropriate modality to use
whenever gait therapy is prescribed for patients who lack the upper and/or lower body strength
to support themselves during assisted ambulation. In addition to aiding gait pattern
regeneration, partial weight bearing therapy allows patients to enhance their endurance,
balance and posture.

20 Biodex Medical Systems, Inc. © 2017
The Biodex FreeStep SAS
The Biodex FreeStep SAS is also ideal for use with the
Biodex Balance System SD.
Biodex FreeStep SAS is an overhead track and harness system that provides a safe ambulation
environment for both therapist and patient. Without the fear of falling, patients can focus more
fully on their tasks of gait and balance. Likewise, therapists can focus on assisting, rather than
supporting. Worn like a vest around the upper torso, the Balance Support Harness provides
patients with security and safety from falling. The versatile harness is supportive, comfortable
and easy to put on and off.
Biodex FreeStep SAS can be custom configured to any facility, complementing the existing
equipment floor plan. Install as a simple loop for continuous ambulation over stairs or through
parallel bars, or add side branches for equipment-specific stations to include treadmill exercise
or balance training.
Figure 4.2. The Biodex FreeStep SAS
This manual suits for next models
2
Table of contents
Other biodex Fitness Equipment manuals

biodex
biodex Balance System SD Series User manual

biodex
biodex 950-401 User manual

biodex
biodex UPPER BODY CYCLE User manual
biodex
biodex Dual Position Back EX User manual

biodex
biodex 950-192 User manual
biodex
biodex SIT2STAND 950-560-10 Installation and operating instructions

biodex
biodex Biostep 2 User manual