biodex Balance System SD Series User manual

BALANCE SYSTEM™ SD (version 4.x)
INSTRUCTIONS FOR USE
950-440 System, Balance SD, 115 VAC 15.6” display
950-441 System, Balance SD, 230 VAC 15.6” display
950-444 System, Balance SD, 100 VAC 15.6” display
950-450 Optional FreeSway Handles
FN: 19-092-CLR Rev B 6/19

2
BALANCE SYSTEM™ SD (version 4.x)
This instructions for use document covers safe operation of the Balance System SD.
Additional information and resources are available upon request or directly from the Biodex
website: www.biodex.com/balance.
Here, the user can find information from compliance to clinical support, and if the desired
information is not found, Biodex can be contacted directly at supportservices@biodex.com.
Thank you,
Biodex Medical Systems, Inc.
Contact information
Manufactured by:
Biodex Medical Systems, Inc.
20 Ramsey Road, Shirley, New York, 11967-4704
Tel: 800-224-6339 (Int’l 631-924-9000)
Fax: 631-924-8355
email: [email protected]
www.biodex.com

3
Table of Contents
Definition of Symbols................................................................................................................ 5!
Product Certifications and Classifications................................................................................... 6!
Warnings and Cautions ............................................................................................................. 8!
Important Safety Information .................................................................................................... 8!
1.!Introduction................................................................................................................. 10!
Intended Use ............................................................................................................... 10!
Indications for Use....................................................................................................... 10!
Contraindications for Use ............................................................................................ 10!
2.!Installation and Configuration ..................................................................................... 12!
Positioning the Balance System SD and Printer Stand ................................................... 12!
Leveling the Base......................................................................................................... 12!
Installing the Printer .................................................................................................... 13!
Installing Optional Accessories .................................................................................... 13!
Assembling the Printer Stand with Optional FreeSway Hooks ....................................... 13!
Turning On the System ................................................................................................ 14!
Turning Off the System................................................................................................ 15!
Configuring the System ............................................................................................... 16!
Common Icons and Buttons ......................................................................................... 19!
3.!Planning a Session ....................................................................................................... 20!
Evaluating a Patient ..................................................................................................... 20!
Setting Up a Session .................................................................................................... 20!
4.!Getting Started ............................................................................................................ 22!
Starting a Session ........................................................................................................ 22!
Adjusting the Support Handles .................................................................................... 22!
FreeSway Handles ........................................................................................................ 23!
Adjusting the Display................................................................................................... 24!
Positioning the Patient’s Feet....................................................................................... 25!
Common Actions ......................................................................................................... 25!
Setting Up a Training Session ...................................................................................... 26!
Setting Up a Test Session............................................................................................. 26!
Saving Results and Creating Reports............................................................................ 27!
5.!Training Modes............................................................................................................ 28!
Percent Weight Bearing Training .................................................................................. 28!
Weight Shift Training ................................................................................................... 29!
Postural Stability Training ............................................................................................ 30!

4
Motor Control Training ................................................................................................ 31!
Maze Control Training ................................................................................................. 32!
Random Control Training............................................................................................. 33!
6.!Testing Modes............................................................................................................. 34!
Sensory Integration Tests ............................................................................................ 35!
Clinical Test of Sensory Integration of Balance (CTSIB) ................................................. 35!
Balance Error Scoring System (BESS)............................................................................. 37!
Limits of Stability (LOS) Test ........................................................................................ 38!
Postural Stability Test .................................................................................................. 39!
Motor Control Test ...................................................................................................... 40!
Bilateral Comparison Test............................................................................................ 41!
Fall Risk Test ............................................................................................................... 42!
7.!Utilities........................................................................................................................ 43!
Reports........................................................................................................................ 44!
Configuration .............................................................................................................. 48!
Change Sensory Integration Defaults ........................................................................... 48!
Changing Fall Risk Defaults ......................................................................................... 50!
Managing Custom Protocols ........................................................................................ 50!
Creating a New Protocol .............................................................................................. 50!
Managing Custom Sensory Tests ................................................................................. 52!
Patient Management .................................................................................................... 54!
8.!VibroTactile™ System................................................................................................... 59!
Getting Started ............................................................................................................ 60!
Attach the Link Box
.................................................................................................... 60!
Connect to the Display
............................................................................................... 61!
Charging the tactile belt receivers................................................................................ 63!
Configuring Equipment in Facilities with One VibroTactile System................................ 67!
Configuring Equipment in Facilities with Multiple VibroTactile Systems........................ 69!
9.!Maintenance ................................................................................................................ 70!
System Maintenance .................................................................................................... 70!
Updating System Software ........................................................................................... 71!
Cleaning the Balance System ....................................................................................... 71!
Maintaining the Printer ................................................................................................ 72!
Disposal ...................................................................................................................... 72!
10.!Specifications and Certifications .................................................................................. 73!

5
Definition of Symbols
The following symbols and their associated definitions are used and implied throughout this
manual.

6
Product Certifications and Classifications
This product has received the following certifications and falls within the following
classifications:
•
ETL Listed Electrical Equipment, Laboratory Use; Part 1, General Requirements for
Safety
conforms to UL 60601-1, CAN/CSA C22.2 No: 601-1-M90, EN 60601-1, EN
60601-1-4
and EN 60601-1-2 and CE Marked.
•
FDA Class II Equipment
•
EC Certificate: EC #4132458
•
Type B Applied Part
•
Electromagnetic Compatibility: This equipment complies with the Medical
Equipment
EN 60601-1-2 EMC Standard.
Mi-Wi Communication Software - Copyright JD Birck, LLC
United States:
Contains FCC ID: OA3MRF24J40MA
Canada:
Contains IC: 7693A-24J40MA
This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential installation. This equipment generates,
uses, and can radiate radio frequency energy, and if not installed and used in accordance with
the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the interference by one or
more of the following measures:
•Reorient or relocate the receiving antenna.
•Increase the separation between the equipment and receiver.
•Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
•Consult the dealer or an experienced radio/TV technician for help.

7
NOTE: Circuit diagrams, component part lists, descriptions, calibration instructions,
or other information used to assist service personnel to repair those parts of the
equipment that are designated as repairable for this product are provided on the
Biodex website, www.biodex.com or can be obtained by contacting Biodex Customer
Service (see Contact information).
Note: Complete information on the Electromagnetic Compatibility for the Biodex Balance
System can be located in the Compliance Supplement located on the Biodex website
(www.biodex.com) or can be obtained by contacting Biodex Customer Service (see Contact
information).
Note: Normative Data for the Balance System is located in the Normative Data Supplement
located on the Biodex website (www.biodex.com) or can be obtained by contacting Customer
Service.
Authorized European Community Representative:
Emergo Europe
Prinsessegracht 20
2514 AP, The Hague
The Netherlands

8
Warnings and Cautions
NOTE: The warnings, cautions, and instructions provided in this manual must be
read, followed, and always kept available for consultation.
CAUTION: Do not modify this equipment without authorization from the
manufacturer. Unauthorized modifications to this system are not permitted and
void the manufacturer’s warranty. Unauthorized modification might result in a
hazard to the user and/or patient.
CAUTION: Federal Law restricts this device to sale by or on the order of a
medical practitioner. When prescribed for therapeutic purpose, a physician must
define the use clearly (for example, total work, maximum heart rate, and so on)
to reduce the risk of patient injury.
Important Safety Information
Read the entire operation manual. Failure to read the manual might result in
user error or inaccurate data. Save all documents for reference.
WARNING
•Allow only qualified, trained personnel to operate or service this system.
•Do not use this system in a manner other than specified in this manual.
If the equipment is used in a non-specified manner, the protection
provided by the equipment might be impaired and results could be
compromised.
WARNING: Never leave patient unattended.
This medical electrical equipment requires special precautions for EMC and must
be installed and used according to EMC information located on the Biodex
website (www.biodex.com).
WARNING: Only use approved power supplies.
CAUTION: Operation for: 115–230 VAC, 50/60 Hz.
CAUTION: To avoid risk of electric shock, this equipment must be connected
only to supply mains with protective earth.
CAUTION: The plug is considered the method of disconnecting the system from
main power. Do not place the system in a position where the plug cannot be
reached quickly.

9
CAUTION: The system is intended to remain in one location during operation.
To relocate the system, the system is disconnected from power source. One
person can move the system. Use the wheels to move the system.
WARNING: Connecting electrical equipment to a multiple socket outlet (MSO)
effectively leads to creating an ME SYSTEM, and can result in a reduced level of
safety.
Patient Weight:
60 lb (27 kg) – 400 lb (181 kg)
Operating Conditions
●Temperature: 15° C to 30° C (60° F to 86° F).
●Humidity Range: 30% to 75% (non-condensing).
●Atmospheric Pressure: 70kpa (10psi) to 106kpa (15psi).
Storage Conditions
●Temperature: -20° C to 60° C (-4° F to 140° F).
●Humidity Range: 10% to 100%.
●Atmospheric Pressure: 50kpa to 101kpa (7psi to 15psi)
Water Resistance Rating: IPX0

10
1. Introduction
Intended Use
The Balance System SD is an assessment tool which allows for testing and training in both static
and dynamic formats. It is a versatile product, providing capabilities for balance assessment,
concussion management, measurement of kinesthetic and proprioceptive abilities, and
determining neuromuscular control (stability and degree of sway values).
Indications for Use
The Balance System SD is indicated to be used for various populations such as neurological
disorders, orthopedic, deconditioned, fall risk, concussion management and other general
conditions.
Contraindications for Use
Contraindications include severe osteoporosis, non-union fractures, Bariatric patients weighing
greater than 400 pounds, patients under 60 pounds, patients with poor safety
awareness/cognition, acute conditions such as pulmonary embolus, thrombus, acute MI, acute
fractures, BP over 180/110 Hg, patients with severe debilitating dizziness.
Parts and Accessories
Figure 1.1. Balance System SD with Printer and Stand:
List of Parts
•Power Cable
•Power Adapter
•Printer Power Cable
•USB Cable
•CTSIB Indexed Pad
•Printer
•Printer Stand
•Wrench
Optional Parts
•
FreeSway Handles
•
USB keyboard or mouse
•
External VGA monitor
•
VibroTactile™ System

11
The optional VibroTactile™ System offers an additional form of sensory feedback. Using
wireless technology, the belt responds with a vibrating sensation when the patient sways
outside the target area. The vibrating feedback is helpful when training requires the patient’s
eyes to be closed.
Figure 1.2. VibroTactile System.
The CTSIB Indexed Pad is a foam pad that is placed over the platform for sensory integration
testing.
Figure 1.3 CTSIB Indexed Pad.
The optional FreeSway Handles are free-floating, yet contained within a controlled opening.
They allow patients to experience unimpeded postural sway while holding onto handles that are
available for support. The FreeSway Handles are ideal for regaining balance and stability.
Figure 1.4 FreeSway Handles.

12
2. Installation and Configuration
The Balance System SD is shipped in a single carton. The System is assembled before shipment
and is ready to operate. The printer requires installation.
Positioning the Balance System SD and Printer Stand
1. Determine the location for equipment.
CAUTION: The plug is the method of disconnecting the system from the
main power. Do not place the system in a position where the plug is not
immediately accessible.
This medical electrical equipment requires special precautions regarding
EMC and must be installed and used according to EMC information.
2. To move the Balance System SD, tip the system and use the wheels to roll the system into
position. When the system is upright, the wheels are not in contact with the floor and the
system is in a fixed position.
3. Position the printer stand next to the Balance System SD.
4. Set the printer on its stand.
Leveling the Base
Figure 2.5 Leveling the Base.
1. Loosen the jam nut using the wrench provided. The wrench is magnetic and can be
stored on the Balance System.
2. Adjust the leveling guide with the wrench until the base is level and stable.
3. Tighten the jam nut so it is flush against the base again.

13
Installing the Printer
Read the printer manual. Locate the USB port and the power inlet.
1. The Balance System SD ships with the USB cable inserted into the display. Insert the other
end of the cable into the USB port of the printer.
2. Insert the printer power cable into the printer power inlet.
3. Insert the printer AC plug into the power adapter.
4. Install the power adapter AC plug into the Balance System SD.
WARNING: Do not connect any other equipment to the Balance System SD power
inlet.
5. Arrange the USB cord and the power cables so they do not interfere with the Balance System
SD platform or support handles.
6. Refer to the printer manual for instructions for managing paper and ink cartridges.
Important Notice Concerning Supplied Printer Ink Cartridges
The printer ink cartridges for the Hewlett-Packard (HP) printer supplied with the Biodex product
may not be compatible for customers outside of the North America, South America, Australia
and some Pacific countries. Do not install the supplied printer cartridges before reading the
enclosed information included with the printer.
Installing Optional Accessories
Locate the necessary cable and power cords for the optional external monitor, keyboard, or
mouse.
1. Connect the monitor VGA cable to the port on the bottom of the system display.
2. Connect the monitor power cord to an AC power supply.
3. Connect the keyboard and mouse cables to the USB ports on the bottom of the system
display.
Assembling the Printer Stand with Optional FreeSway Hooks
When the printer stand is not in use, it can be used to hang the Optional FreeSway Handles.
Refer to the printer stand drawing in the Parts and Assembly Illustrations appendix.
1. Remove hooks and all hardware from packaging.
2. Apply Loctite to the nut.
3. Put the nut onto the hook.
4. Place the washer on top of the nut.
5. Insert hook into the bottom of the stand.

14
6. Insert finishing washer over the stud.
NOTE: The flange should be sticking up.
7. Apply Loctite to second nut.
8. Thread on the second nut and tighten.
NOTE: Ensure the hooks are orientated correctly, pointing outwards as shown in the
diagram before tightening.
9. Apply plastic cap.
10. Repeat steps 1-9 for the remaining three hooks.
Turning On the System
The Balance System SD turns on when the power cord is plugged into an AC power supply. The
software starts automatically.
1. Plug the power cord of the Balance System SD into an AC power supply. The Home screen is
displayed (see Figure 2.1). The Balance System platform is active and in the locked position.
Figure 2.1. Home Screen .
2. Turn on the printer.
3. If an external monitor is used, turn on the monitor. At the Home screen, select <Utilities >
<Configuration >, <System Configuration >, <Screen Configuration >, and <Mirror to
External Monitor>.

15
Turning Off the System
CAUTION: Shut down the software before unplugging the device.
To preserve the Balance System SD database, use the proper sequence to turn off the system:
1. Shut down the software as illustrated in Figure 2.2.
2. Unplug the system from the power supply.
Figure 2.2. Shut Down Icon.
The software can be restarted while the system is plugged in. Touch the rocker switch on the
bottom of the display to restart the software (see Figure 2.3).
Figure 2.3. Power Switch on Display.
1
2

16
Configuring the System
1. Touch <Utilities> on the Home screen.
2. At the Access ID Code screen, enter the ID code 159 and touch <OK>.
3. Touch <Configuration>.
4. Touch <System Configuration>
5. Select display options and set values for actions (see Figure 2.4).
Figure 2.4. System Configuration Screen.
Table 2.1 System Configuration Functions.
Function
Default
Options
<Test Completion Screen Time Out>
Set the number of minutes to display the
test results after a test.
One minute
Touch the <▲> or <▼> keys to
increase or decrease to any time
from 0:00 to 30:00 minutes.
<Tone Volume>
Set the volume for the sound emitted
each time the patient makes the cursor
hit a target.
On
Touch any section of the bar to set
the volume level from 1 to 10.
<Screen Configuration>
The Screen Saver setting sets the
number of minutes the display remains
on when the system is not in use. After
the inactivity timer expires, the screen
fades.
Inactive
1. Touch the <> or <> keys to
set the number of minutes of
inactivity.
2. To activate, touch the <Enable
Screen Saver> box. The box
color changes to green.
The user can view the same contents of
the display on an external monitor.
No external
monitor
1. Check that the external monitor
is plugged into the system
display and a power supply. Turn
on the monitor.
2. Touch <Enable/Disable External
Monitor>. The external monitor
mirrors the display.
3. Touch <OK> to return to the
Configuration screen.

17
Function
Default
Options
<Set Date/Time>
Touch <Set Date/Time> to change the
system time or date.
Eastern
Standard
Time (EST)
1. Touch to highlight the value
being changed, and use the
<> or <> keys to increase or
decrease the value.
2. Touch <OK> to accept the
changes and return to the
Configuration screen.
<Units>
The device can use either US standard or
metric measurements.
US
Touch the arrow on the Unit
dropdown list, and then touch the
type of unit.
<Change Access ID Code>
The code restricts access to certain
sections of the system configuration
such as default system settings and
patient data.
159
1. To change the access ID code,
touch the keypad icon, and
enter the new code.
2. Touch <OK> to save the New
Access ID Code and return to
the Default Settings screen.
6. After changes have been made, touch <Back> to exit and return to the Utilities menu.
7. Touch <Back> a second time to return to the Home Menu.
8. To complete the configuration:
•
Touch <Utilities> on the Main screen.
•
At the Access ID Code screen, enter the ID code 159 and touch <OK>.
•
Touch <Configuration>.
•
Touch Balance System SD Configuration screen (see Figure 2.5).
Figure 2.5. Balance System SD Configuration Screen.

18
Table 2.2 Balance SD Configuration Functions Table.
Function
Default
Options
<Clinical Codes>
Not applicable
Not applicable
<Sensory Integration
Defaults>
mCTSIB default data
No BESS data
Specify an alternative data
set for mCTSIB or create BESS
data-.
Refer to Sensory Integration
Tests.
<Fall Risk Defaults>
Refer to Error! Reference
source not found..
Changing the default
Changing the default
conditions makes the
comparisons to the
normative data invalid.
<Custom Protocol List>
None
Refer to Managing Custom
Protocols.
<Facility Information>
Information about the site.
None
Touch <Facility Information>
to enter text about the site.
Touch <Print Facility
Information on Reports> to
include site information in
reports.
Enable Tone
Disabled
Touch the check box to
enable audio feedback.
Set the volume of the tone in
<Tone Volume>.
Enable Advanced Data Input
Mode
Patient Setup requires:
•Age
•Height Range
Touch the check box to
require:
•Date of Birth
•Height
Require Patient ID# for Patient
Record
Patient Setup does not
use a patient ID number
or medical record
number.
Touch the check box to
require a Patient ID#.
Enable ‘Additional Information’
on Patient Setup
Patient Setup has an
inactive Additional Info
icon.
Touch the check box to
enable the Additional Info
icon. This allows users to
further specify the patient
profile with information such
as Health Status, Alternate
ID, Group, Sport, Facility,
and Referred By. Up to four
more fields can be added,
customized for the site.
Enable VibroTactile feedback
Disabled. Refer to
VibroTactile System.
Touch the check box if the
VibroTactile System is used
with the Balance System SD.
<Reporting>
Enable Impairment %
None
Touch the check box to input
a G-code for Patient Setup.
Print Facility Information on
Reports
Disabled
Touch the check box to input
information about the site
that will be displayed on
printed reports.
Print Custom Logo on Reports
Disabled
Touch the check box to
display the logo on reports.

19
Common Icons and Buttons
Table 2.3. Action Table for Configuration Function Icons.
Icon
Name
Action
Home
Returns to the Main Menu.
Next
Advances to the next screen.
Back
Returns to the previous screen.
OK
Confirms changes and advances to the next screen.
Clear Tracing
To erase the trace and start again, touch the <Clear Tracing> icon.
Magnifying
Glass
Enlarges the screen for easier viewing. After enlarging the view,
the setting for the platform cannot be changed until the original
view is restored.
Keypad
Displays a window to enter numbers.
Keyboard
Displays a window to enter text.
Start
Begins the session and the timer starts. The current score is
displayed.
Stop
Stops the session. No more data is recorded, and the platform
locks in the static position.
Touch <Start> to begin another session using the same
parameters.

20
Figure 3.1. Patient Setup Screen.
3. Planning a Session
For a productive patient session, practice the adjustments and display selections that will be
needed, and review the patient history.
Evaluating a Patient
•A postoperative patient must possess adequate muscular control to stabilize the joint.
Inadequate muscular control might lead to increased joint translation.
•A patient’s supporting structures above and below the joint must have adequate strength.
Setting Up a Session
For a new patient, create a patient record. For a returning patient, retrieve the patient record.
NOTE: Specifying the patient’s height is mandatory because height is used to calculate the
patient’s center of gravity (COG) when the platform is in a level, static mode. The
height or weight cannot be entered or changed after a test is complete.
Setting Up a Current Session
Setting Up a Future Session
Touch either <Testing> or <Training> to
display the Patient Setup screen (see Figure
3.1).
Touch Utilities > Patient Management.
At the Access ID Code screen, enter the ID
code 159 or the site’s code, and touch <OK>.
Touch <Add Patient> (see Figure 3.2).
Figure 3.2. Add Patient Screen.
For a New Patient:
1. Touch the keyboard or keypad icon to enter text and values in the fields.
2. If the Patient Setup screen is being used, touch the appropriate Height icon for this patient.
3. Touch the appropriate gender icon.
4. If the Additional Information icon is enabled, touch the icon to display the Additional
Information screen, as illustrated in Figure 3.3. Enter the information required by the site
and touch <OK>.
This manual suits for next models
4
Table of contents
Other biodex Fitness Equipment manuals
biodex
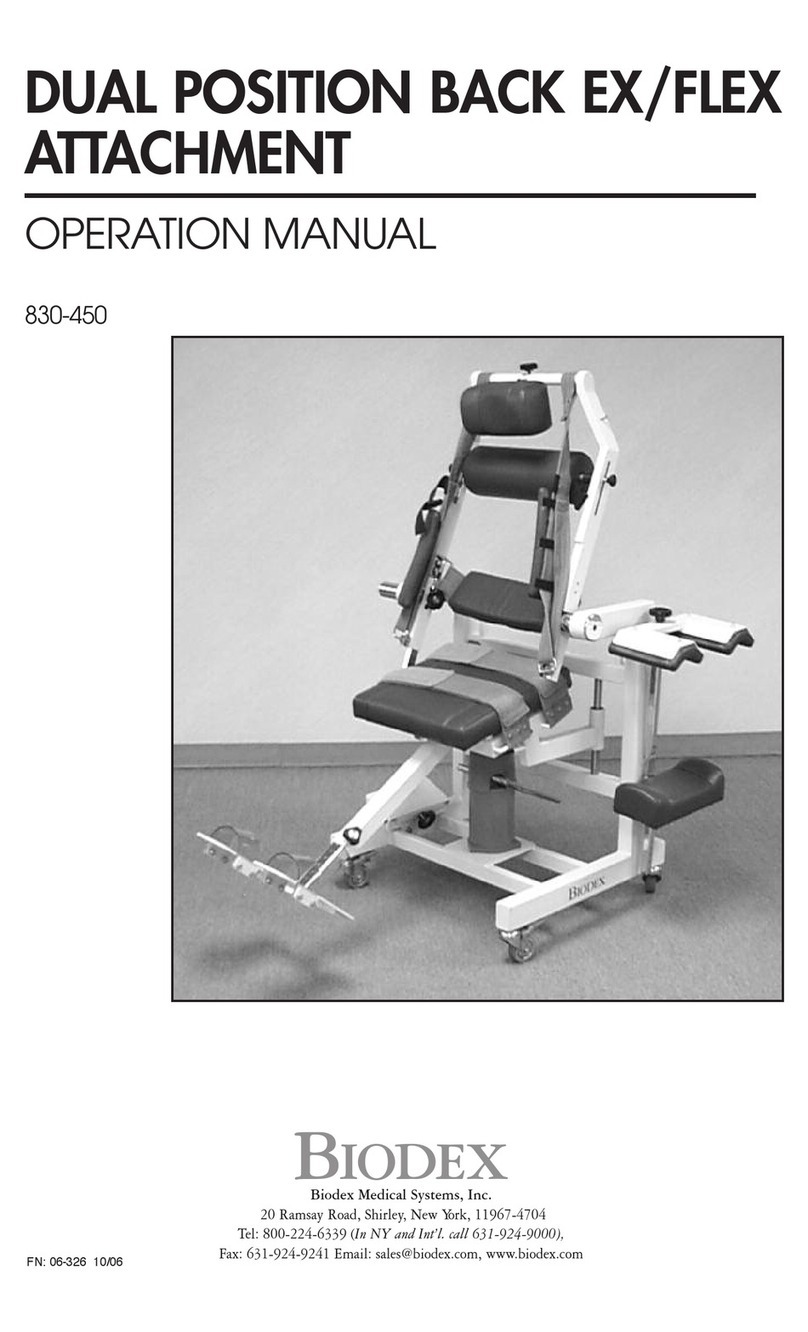
biodex Dual Position Back EX User manual
biodex
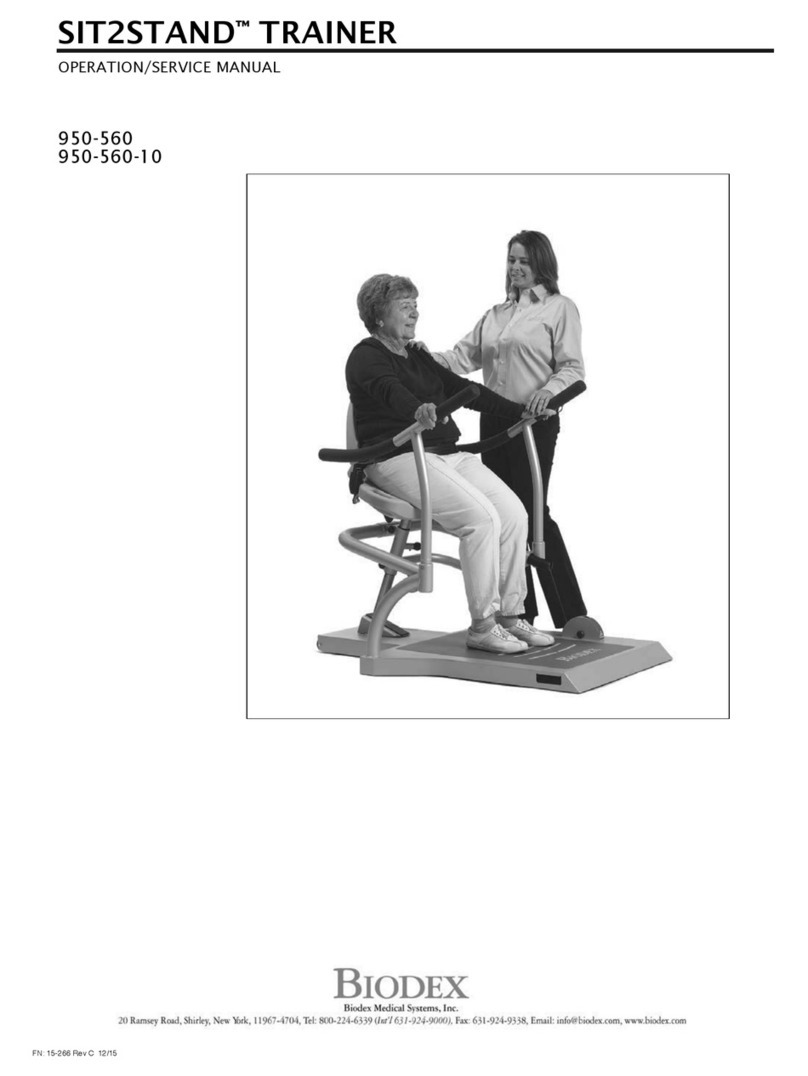
biodex SIT2STAND 950-560-10 Installation and operating instructions

biodex
biodex 950-192 User manual

biodex
biodex Biostep 2 User manual

biodex
biodex 950-440 Installation and operating instructions

biodex
biodex 950-401 User manual

biodex
biodex UPPER BODY CYCLE User manual
Popular Fitness Equipment manuals by other brands

G-FITNESS
G-FITNESS AIR ROWER user manual

CAPITAL SPORTS
CAPITAL SPORTS Dominate Edition 10028796 manual

Martin System
Martin System TT4FK user guide

CIRCLE FITNESS
CIRCLE FITNESS E7 owner's manual

G-FITNESS
G-FITNESS TZ-6017 user manual

Accelerated Care Plus
Accelerated Care Plus OMNISTIM FX2 CYCLE/WALK user manual











