Bioness NESS L300 User manual

NESS L300®
User’s Guide

L300 User’s Guide Copyright
© 2014, Bioness Inc.
All Rights Reserved
No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or
translated into any language or any computer language, in any form or by any third party, without the prior
written permission of Bioness Inc.
Trademarks
NESS®, NESS L300®, Intelli-Gait®, Intelli-Sense Gait Sensor™, Bioness, the Bioness Logo® and LiveOn® are
trademarks of Bioness Inc. in the United States or other countries. | www.bioness.
Rx Only (US Only)
Patents
This product is covered by one or more US and international patents. Other patents pending.
Disclaimer
Bioness Inc. and its affiliates shall not be liable for any injury or damage suffered by any person, either directly
or indirectly, as a result of the unauthorized use or repair of Bioness Inc. products. Bioness Inc. does not
accept any responsibility for any damage caused to its products, either directly or indirectly, as a result of use
and/or repair by unauthorized personnel.
Environmental Policy
Service personnel are advised that when changing any part of the NESS L300 System, care should
be taken to dispose of those parts in the correct manner; where applicable, parts should be recycled.
For more detailed information regarding these recommended procedures, please contact Bioness Inc.
Bioness Inc. is committed to continuously seeking and implementing the best possible manufacturing
procedures and servicing routines.
Worldwide Corporate Office
Bioness Inc
25103 Rye Canyon Loop
Valencia, CA 91355 USA
Telephone: 800-211-9136 or
661.362.4850
Email: [email protected]
Website: www.bioness.com
Manufactured by
Bioness Neuromodulation Ltd.
19 Ha’Haroshet Street
PO Box 2500
Industrial Zone
Ra’Anana 43654, Israel
European Authorized Representative
NESS Europe B.V.
Stationsweg 41
3331 LR Zwijndrecht, The Netherlands
Telephone: +31.78.625.6088
Email: [email protected]
Website: www.bioness.com
Conformity Certification

III
List of Symbols
Caution
Warning
Double Insulated (Equivalent to Class II of IEC 536)
Type BF Applied Part(s)
Non-Ionizing Radiation
Date of Manufacture
Manufacturer
This Product Must Not Be Disposed of with Other Household Waste
Refer to Instruction Manual/ Booklet
Re-Order Number
Lot Number
Serial Number
Complies with United States and Canadian Product Safety Standards
Single Patient Use
Storage Temperature
Complies with the European Union Medical Device Directive
European Authorized Representative
Humidity Limitation
Atmospheric Pressure Limitation
Keep Dry

IV
Table of Contents
List of Symbol .................................................................................................... iii
Chapter 1: Introduction ........................................................................ 1
Chapter 2: Safety Information .............................................................. 3
Indications for Use ............................................................................................. 3
Contraindications ............................................................................................... 3
Warnings ........................................................................................................... 3
Precautions ........................................................................................................ 4
Adverse Reactions ............................................................................................ 6
Skin Care Guidelines ......................................................................................... 6
Chapter 3: Environmental Conditions that Affect Use ....................... 9
Radio Frequency (RF) Communication ............................................................. 9
Travel ................................................................................................................. 10
Chapter 4: L300 System Kit .................................................................. 11
Contents ............................................................................................................ 11
Chapter 5: Device Description ............................................................. 15
Functional Stimulation (FS) Cuff ....................................................................... 15
L300 RF Stim Unit ............................................................................................. 15
Electrodes and Electrode Bases ....................................................................... 17
Intelli-Sense Gait Sensor ................................................................................... 19
Control Unit ........................................................................................................ 20
Operating Modes ......................................................................................... 20
Operating Buttons ........................................................................................ 21
Digital Display and Indicator Lights ............................................................. 22
Audio Indicators ........................................................................................... 23
Chapter 6: Setup Instructions .............................................................. 25
Charging the L300 System ................................................................................ 25
Preparing the Skin ............................................................................................. 27

V
Attaching the Electrodes .................................................................................... 27
L300 Quick Fit Electrode ............................................................................. 27
L300 Cloth Electrodes ................................................................................. 28
L300 Hydrogel Electrodes ........................................................................... 29
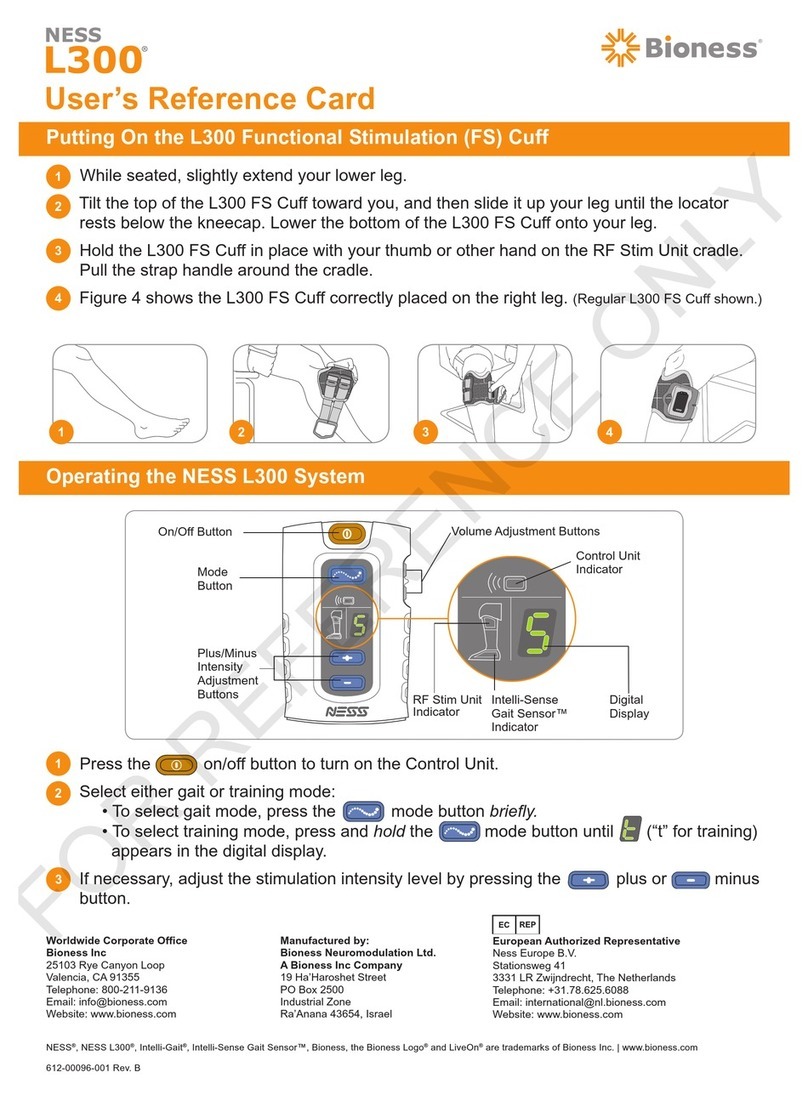
Positioning the L300 FS Cuff ............................................................................. 30
Removing the L300 FS Cuff .............................................................................. 32
Positioning the Intelli-Sense Gait Sensor .......................................................... 32
Chapter 7: Operating the L300 System ............................................... 35
RF Communication Safety Features ................................................................. 35
Turning the L300 System On/Off ....................................................................... 35
Selecting an Operating Mode ............................................................................ 35
Gait Mode .................................................................................................... 35
Training Mode .............................................................................................. 36
Standby Mode ............................................................................................. 36
Adjusting Stimulation Intensity ........................................................................... 36
Adjusting the Volume of Audio Alerts ................................................................. 37
Turning On Audio Feedback During Stimulation .......................................... 37
Chapter 8: Maintenance and Cleaning ................................................ 39
Daily Maintenance and Storage ........................................................................ 39
Charging ............................................................................................................ 39
Battery Replacement: L300 RF Stim Unit .......................................................... 39
Battery Replacement: Intelli-Sense Gait Sensor ............................................... 40
Battery Replacement: Control Unit .................................................................... 41
Replacing the L300 Quick Fit Electrodes .......................................................... 42
Replacing the Cloth Electrodes ......................................................................... 44
Replacing the Hydrogel Electrodes ................................................................... 45
Replacing the Electrode Bases ......................................................................... 47
Removing the RF Stim Unit ............................................................................... 49

VI
Inserting the RF Stim Unit ................................................................................. 49
Cleaning Your L300 System Components ......................................................... 50
Disinfecting ........................................................................................................ 51
Chapter 9: Electronic Registration of Replacement 0ARTS¬ ................ 53
Registering a New Control Unit ......................................................................... 53
Registering a New RF Stim Unit ........................................................................ 55
Registering a New Intelli-Sense Gait Sensor .................................................... 57
Chapter 10: Troubleshooting ................................................................ 59
Chapter 11: Technical Specifications .................................................. 65
Chapter 12: Appendix - EMI Tables ...................................................... 71

VII User’s Guide
This page intentionally left blank.

1
Introduction
Central nervous system injuries often cause a gait disorder called foot drop. People who
have foot drop are unable to raise their foot while walking. They often drag their foot,
resulting in instability and increased effort during gait.
The NESS L300 Foot Drop System is an advanced neuroprosthesis designed to improve gait
in people suffering from foot drop. The L300 System incorporates cutting-edge technology
and sophisticated design features to improve walking and quality of life.
The L300 System consists of a Functional Stimulation (FS) Cuff (available in regular and
small sizes) with a Radio Frequency (RF) Stim Unit, an Intelli-Sense Gait Sensor, and
a Control Unit. These components communicate wirelessly to send electrical pulses to
the peroneal nerve, which controls the muscles of the lower leg. When stimulated at the
appropriate phase of walking, the muscles raise the foot, thereby preventing foot drop.
Chapter 1 - Introduction
1
Functional Stimulation Cuff with Radio
Frequency Stim Unit
Intelli-Sense
Gait Sensor Control Unit
Regular L300
FS Cuff Small L300 FS Cuff

2User’s Guide
System Features:
• The FS Cuff includes a cradle for the RF Stim Unit and an ergonomic locator to
ensure constant, snug contact with the leg. The FS Cuff can be put on with one hand.
• The Intelli-Sense Gait Sensor can detect when the foot is in the air and on the
ground and regulate stimulation appropriately.
• The hand-held Control Unit monitors system status and manages system performance.
This L300 User's Guide describes:
• Important safety information about the L300 System.
• The components of the L300 System.
• How to set up, operate, and maintain your L300 System.
• Troubleshooting information.
Your clinician has prescribed the L300 System to treat your foot drop. Be sure to read this
guide before using your L300 System. Be sure to review this guide with your clinician. If you
have any questions contact the Bioness Client Relations Department at (800) 211-9136,
Option 3 (in the United States) or your local distributor (outside of the United States). You
can also visit the Bioness website at: www.bioness.com.
Caution: Do not put on or operate the L300 System before being properly fitted and
trained by a certified clinician.

3
Safety Information
Indications for Use
The NESS L300 Foot Drop System is intended to provide ankle dorsiflexion in individuals
(adults and pediatrics) who have foot drop following an upper motor neuron injury or
disease. During the swing phase of gait, the NESS L300 electrically stimulates muscles
in the affected leg to provide dorsiflexion of the foot. The NESS L300 may improve gait,
facilitate muscle re-education, prevent or retard disuse atrophy, maintain or increase joint
range of motion, and increase local blood flow.
Contraindications
• Patients with a demand-type cardiac pacemaker, defibrillator, or any electrical or
metallic implant should not use the L300 System.
• The L300 should not be used where a cancerous lesion is present or suspected.
• The L300 FS Cuff should not be used on a leg where a regional disorder, such as a
fracture or dislocation, could be adversely affected by motion from the stimulation.
• The L300 FS Cuff should not be used on a leg where strength testing or strength
training is planned.
Warnings
• The long-term effects of chronic electrical stimulation are unknown.
• The L300 FS Cuff should not be worn over swollen, infected, or inflamed areas or
skin eruptions, such as phlebitis, thrombophlebitis, and varicose veins.
• Simultaneous connection of the L300 System to the patient and high-frequency
surgical equipment may result in skin burns where the stimulator electrodes adhere
and damage to the RF Stim Unit.
• Do not use the L300 System within three feet of short wave or microwave therapy
equipment. Such equipment may produce instability in the RF Stim Unit output.
• The L300 System should only be configured by an authorized clinician.
Chapter 2 - Safety Information
2

4User’s Guide
• In case of any inconvenience, turn off stimulation and remove the L300 FS Cuff. If
the stimulation cannot be turned off, remove the L300 FS Cuff to stop stimulation.
Precautions
• Inflammation in the region of the L300 FS Cuff may be aggravated by motion,
muscle activity, or pressure from the L300 FS Cuff. Stop using the L300 System until
the inflammation is gone.
• Use caution if you have a suspected or diagnosed heart problem.
• Use the L300 FS Cuff with caution:
• If you have a tendency to hemorrhage following acute trauma or fracture.
• Following recent surgical procedures when muscle contraction may disrupt the
healing process.
• Over areas of the skin that lack normal sensation.
• If you have suspected or diagnosed epilepsy.
•
Some patients may experience a skin irritation, an allergic reaction, or hypersensitivity
to the electrical stimulation or the electrical conductive medium. Irritation may be
avoided by having your clinician change the stimulation parameters, type of
electrodes, or electrode placement.
• Do not use the L300 System without electrodes.
• After removing the L300 FS Cuff, it is normal for the areas under the electrodes to be
red and indented. The redness should disappear in approximately one hour. Persistent
redness, lesions, or blisters are signs of irritation. Alert your clinician and stop using
the L300 System until any inflammation is gone.
•
Stop using the L300 System and consult your clinician if stimulation does not start at
the correct time during gait.
• Do not wear the L300 System during x-ray examinations.
• Turn off the L300 System when at a refueling place. Do not use the L300 System
near flammable fuel, fumes, or chemicals.
• Only your treating clinician should determine electrode placement and stimulation
settings.

5
Chapter 2 - Safety Information
• Use only the L300 System electrodes supplied by Bioness Inc.
• Turn off the L300 System before removing or replacing the electrodes.
• Obtain physician clearance prior to use if you have an alteration in normal arterial
or venous flow in the region of the L300 FS Cuff because of local insufficiency,
occlusion, arteriovenous fistula for hemodialysis, or a primary disorder of the
vasculature.
• Obtain physician clearance before stimulating an area with a structural deformity.
• The safe use of the L300 System during pregnancy has not been established.
• Skin problems, on the leg where the FS Cuff is worn, may be aggravated by the
L300 System.
• Adult supervision and assistance should be provided for anyone needing help while
using the L300 system.
• The Control Unit neck strap is meant to be worn around the neck and if not used
properly could cause bodily harm.
• Protect all electronic components from contact with water, such as from sinks,
bathtubs, shower stalls, rain, snow, etc.
•
Do not leave the L300 System stored where temperatures may exceed the acceptable
environmental range: -20°C to +60°C (-4°F to +140°F). Temperature extremes can
damage the components.
• Do not attempt to repair your L300 System. Contact Bioness if you experience a
technical problem not covered in this guide.
• The L300 FS Cuff is to be worn only on the leg of the patient for whom it is fitted. It
should not be worn by anyone else or on any other part of the body.
• Turn off the L300 System before putting on the L300 FS Cuff. Do not turn on the
L300 System until the L300 FS Cuff is fastened in place.
• Shut off the L300 System before driving, operating machinery, or performing any
activity in which involuntary muscle contractions could injure you.
• Protect the L300 System electronic components from condensation. When moving
the components between hot and cold temperatures, place them in an airtight plastic
bag, and let them slowly (for at least two hours) adjust to the temperature change
before use.

6User’s Guide
• Medical electrical equipment needs special precautions for electromagnetic compatibility.
• Remove the L300 System before undergoing any diagnostic or therapeutic medical
procedure such as x-ray examination, ultrasound, MRI, etc.
Adverse Reactions
In the unlikely event that any of the following occurs, stop using your L300 System immediately
and consult your physician.
• Signs of significant irritation or pressure sores where the L300 FS Cuff contacts the skin.
• A significant increase in muscle spasticity.
• A feeling of heart-related stress during stimulation.
• Swelling of the leg, knee, ankle, or foot.
• Any other unanticipated reaction.
Skin irritations and burns have been reported with the use of powered muscle stimulators.
Skin Care Guidelines
In the absence of proper skin care, extended use of electrical stimulation may cause skin
irritation or a skin reaction to the electrodes or the L300 FS Cuff. Skin irritation tends to
occur after approximately three months of use. To promote healthy skin with long-term use
of the L300 System, it is important to follow a daily skin-care routine.
• Clean the skin where the electrodes adhere with a wet washcloth. If any oils or
lotions are on the skin, then clean with soap and water. Rinse well.
• Always check the skin for redness or a rash when putting on and taking off the L300
FS Cuff.
• Replace the electrodes at least every two weeks, even if they appear to be in good
condition.
• After taking off the L300 FS Cuff, always re-cover hydrogel electrodes with the
protective plastic covers, where applicable.

7
Chapter 2 - Safety Information
• Excess body hair where the electrodes adhere may reduce electrode contact with
the skin. If necessary, remove excess body hair with an electric shaver or scissors.
Do not use a razor. A razor can irritate the skin.
• When positioning the L300 FS Cuff, make sure the electrodes uniformly contact the
skin.
• Ventilate the skin by removing the L300 FS Cuff for at least 15 minutes every three
to four hours.
If skin irritation or a skin reaction occurs, stop using your L300 System immediately and
contact your clinician or dermatologist. Also contact the Bioness Client Relations Department:
(800) 211-9136, Option 3 (in the United States) or your local distributor (outside the United
States). Resume use only when the skin is completely healed, and then follow a skin
conditioning protocol per the recommendation of your health-care specialist.

8User’s Guide
This page intentionally left blank.

9
Environmental Conditions that Affect Use
Radio Frequency (RF) Communication
Several components of the L300 System communicate via radio communication and have
been tested and found to comply with the limits for a Class B digital device, pursuant to
Part 15 (RF Devices) of the Federal Communications Commission (FCC) Rules. These
limits are designed to provide reasonable protection against harmful interference in a
residential installation. This equipment generates, uses, and can radiate RF energy and, if
not installed and used in accordance with the instructions, may cause harmful interference
to radio communications. However, there is no guarantee that interference will not occur
in a particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the user
is encouraged to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation distance between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for assistance.
The antenna for each transmitter must not be co-located or operating in conjunction with
any other antenna or transmitter.
Portable and mobile RF communications equipment can affect the L300 System.
Conformity Certification
The L300 System complies with Part 15 of the FCC rules. Operation is subject to the
following two conditions:
1. This device may not cause harmful interference, and
2. This device must accept any interference received, including interference that may
cause undesired operation.
Chapter 3 - Environmental Conditions that Affect Use
3

10 User’s Guide
Travel
The L300 System charger set with interchangeable blades is compatible with Australian,
U.K., European Union, and U.S. voltage: 110-220 V, 50/60 Hz.
Turn off your L300 system before going through airport security. Wear loose clothing so that
you can easily show the security person your L300 System. The L300 System will likely
set off the security alarm. Either ask for a “hand scan” or be prepared to remove your L300
System so that security can scan it. You may want to carry a copy of your L300 prescription.
A prescription can be useful when passing through customs as well. To request a copy of
your prescription, call the Bioness Client Relations Department at (800) 211-9136, Option
3; or your local distributor.
Note: The L300 System contains radio transmitters. The Federal Aviation Administration
(FAA) rules require that all radio-transmitting devices be turned off during flight.
Electromagnetic Emissions
The L300 System needs special precautions regarding electromagnetic compatibility (EMC)
and needs to be installed and put into service according to the EMC information provided
in this manual.
The L300 System was tested and certified to use the following:
• DC power supply as provided by Bioness Inc, manufactured by Friwo, Part No.
FW7555M/05.
• "Y" cable (2-way splitter) as provided by Bioness Inc. Manufactured by Tamuz
Electronics Ltd.
Warnings
• The use of accessories, transducers, and cables other than those specified, with
the exception of transducers and cables sold by the manufacturer of the L300
System as replacement parts for internal components, may result in increased
emissions or decreased immunity of the L300 System.
•
The L300 System should not be used adjacent to or stacked with other equipment. If
adjacent or stacked use is necessary, the equipment or system should be observed
to verify normal operation in the configuration in which it will be used.

11
Chapter 4 - L300 System Kit
4
L300 System Kit
Contents
Small L300 System Kit
• Small L300 FS Cuff, Right or Left, with (XS) Strap
• L300 RF Stim Unit
• Intelli-Sense Gait Sensor
• Control Unit
• System Charger Set
• Small L300 FS Cuff Strap (XXS)
• Gait Sensor Pads
• Shoe Spacers
• Gait Sensor Replacement Battery
• Small Electrode Bases
• Cloth Electrode Mesh Bag
• Control Unit Neck Strap
• Control Unit Wrist Strap
• Control Unit Belt Pouch
• Phillips Screwdriver
• User’s Guide
• User's Reference Card

12 User’s Guide
Regular L300 System Kit
• Regular L300 FS Cuff, Right or Left, with (M) Strap
• L300 RF Stim Unit
• Intelli-Sense Gait Sensor
• Control Unit
• System Charger Set
• Regular L300 FS Cuff Strap (S)
• Regular L300 FS Cuff Strap (L)
• Gait Sensor Pads
• Shoe Spacers
• Gait Sensor Replacement Battery
• Cloth Electrode Mesh Bag
• Control Unit Neck Strap
• Control Unit Wrist Strap
• Control Unit Belt Pouch
• Phillips Screwdriver
• User’s Guide
• User's Reference Card

13
Chapter 4 - L300 System Kit
Control Unit System Charger Set
Intelli-Sense Gait Sensor
Regular FS Cuff Strap
(S, M, L)
Small FS Cuff Strap
(XXS, XS)
Regular L300 FS Cuff
and RF Stim Unit
Small L300 FS Cuff
and RF Stim Unit
Other manuals for NESS L300
4
Table of contents
Other Bioness Personal Care Product manuals