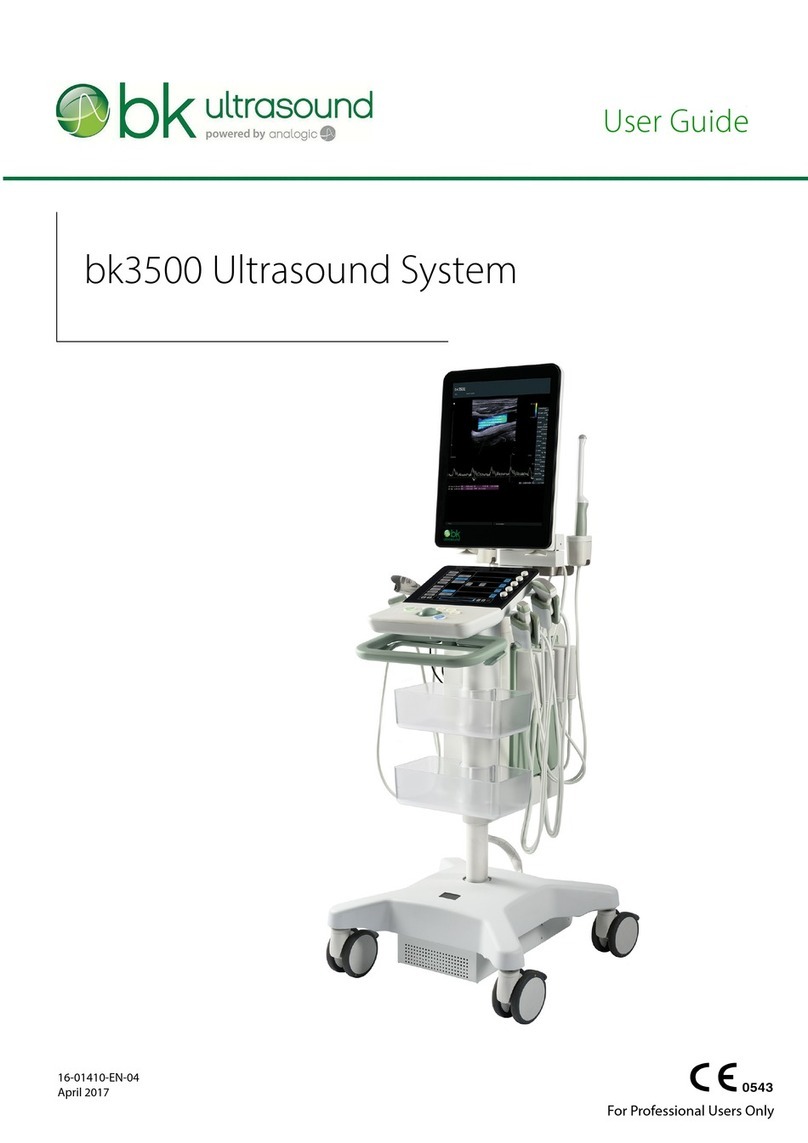
bk ultrasound bk3000 User manual

16-01249-EN-0116-01249-EN-01
January 2015January 2015
For Professional Users OnlyFor Professional Users Only
bk3000 Ultrasound Systembk3000 Ultrasound System

LEGAL MANUFACTURERLEGAL MANUFACTURER
BK Medical ApsBK Medical Aps
Mileparken 34Mileparken 34
2730 Herlev2730 Herlev
DenmarkDenmark
TelTel
.:+45 4452 8100/Fax:+45 4452 .:+45 4452 8100/Fax:+45 4452
81998199
www.analogicultrasound.comwww.analogicultrasound.com
Email: info@analogicultrasound.comEmail: info@analogicultrasound.com
The serial number label on a BK Medical product contains information about the year of manufacture.The serial number label on a BK Medical product contains information about the year of manufacture.
BK Medical Customer SatisfactionBK Medical Customer Satisfaction
Input from our customers helps us improve our products and services. As part of our customerInput from our customers helps us improve our products and services. As part of our customer
satisfaction program, we contact a sample of our customers a few months after they receive their orders.satisfaction program, we contact a sample of our customers a few months after they receive their orders.
If you receive an email message from us asking for your feedback, we hope you will be willing toIf you receive an email message from us asking for your feedback, we hope you will be willing to
answer some questions about your experience buying and using our products. Your opinions areanswer some questions about your experience buying and using our products. Your opinions are
important to us. You are of course always welcome to contact us via your BK Medical representative orimportant to us. You are of course always welcome to contact us via your BK Medical representative or
by contacby contac
ting us directing us direc
tlytly
..
If you have comments about the user documentation, please write to us at the email address above. WeIf you have comments about the user documentation, please write to us at the email address above. We
would like to hear from you.would like to hear from you.
System SoftwareSystem Software
••
NOT FAULT TOLERANTNOT FAULT TOLERANT
. THE SOFTWARE IS NOT FAULT TOLERANT. BK Medical HAS. THE SOFTWARE IS NOT FAULT TOLERANT. BK Medical HAS
INDEPENDENTLY DETERMINED HOW TO USE THE SOFTWARE IN THE DEVICE, AND MS HASINDEPENDENTLY DETERMINED HOW TO USE THE SOFTWARE IN THE DEVICE, AND MS HAS
RELIED UPON BK Medical TO CONDUCT SUFFICIENT TESTING TO DETERMINE THAT THERELIED UPON BK Medical TO CONDUCT SUFFICIENT TESTING TO DETERMINE THAT THE
SOFTWARE IS SUITABLE FOR USE.SOFTWARE IS SUITABLE FOR USE.
••
EXPORT RESTRICTIONSEXPORT RESTRICTIONS
. You acknowledge that Windows 8 Embedded is . You acknowledge that Windows 8 Embedded is
of US-origin. You agree to complyof US-origin. You agree to comply
with all applicable international and with all applicable international and
national laws that apply to Windows 8 Embedded, including the U.S. Exportnational laws that apply to Windows 8 Embedded, including the U.S. Export
Administration Regulations, as well as end-user, end-use and country destination restrictions issued by U.S. andAdministration Regulations, as well as end-user, end-use and country destination restrictions issued by U.S. and
other governments. For additional information on exporting Windows 8 Embedded, see http://other governments. For additional information on exporting Windows 8 Embedded, see http://
www.microsoft.com/exporting/www.microsoft.com/exporting/
••
The bk3000 Ultrasound System is closed. Any modification of or installation of software to the system mayThe bk3000 Ultrasound System is closed. Any modification of or installation of software to the system may
compromise safety and function of the system. Any modification of or installation of software without writtencompromise safety and function of the system. Any modification of or installation of software without written
permission from permission from
BK BK
Medical wMedical w
ill immill imm
ediately void ediately void
any warrantany warrant
y supplied y supplied
by BK by BK
Medical. Such Medical. Such
changes will changes will
alsoalso
void any service contract and result in charges to the customer for restoration of the original bk3000 Ultrasoundvoid any service contract and result in charges to the customer for restoration of the original bk3000 Ultrasound
System.System.
Trademarks:Trademarks:
DICOM is the registered trademark of the DICOM is the registered trademark of the
National Electrical Manufacturers Association for its standards National Electrical Manufacturers Association for its standards
publicationspublications
relating to digital communications of medical information.relating to digital communications of medical information.
Microsoft and Windows are registered trademarks of Microsoft Corporation in the United States and other countries.Microsoft and Windows are registered trademarks of Microsoft Corporation in the United States and other countries.
bk3000 = Ref. Type 2300bk3000 = Ref. Type 2300
© 2015 BK Medical© 2015 BK Medical
Information in this document may be subject to change without notice.Information in this document may be subject to change without notice.

LEGAL MANUFACTURERLEGAL MANUFACTURER
BK Medical ApsBK Medical Aps
Mileparken 34Mileparken 34
2730 Herlev2730 Herlev
DenmarkDenmark
TelTel
.:+45 4452 8100/Fax:+45 4452 .:+45 4452 8100/Fax:+45 4452
81998199
www.analogicultrasound.comwww.analogicultrasound.com
Email: info@analogicultrasound.comEmail: info@analogicultrasound.com
The serial number label on a BK Medical product contains information about the year of manufacture.The serial number label on a BK Medical product contains information about the year of manufacture.
BK Medical Customer SatisfactionBK Medical Customer Satisfaction
Input from our customers helps us improve our products and services. As part of our customerInput from our customers helps us improve our products and services. As part of our customer
satisfaction program, we contact a sample of our customers a few months after they receive their orders.satisfaction program, we contact a sample of our customers a few months after they receive their orders.
If you receive an email message from us asking for your feedback, we hope you will be willing toIf you receive an email message from us asking for your feedback, we hope you will be willing to
answer some questions about your experience buying and using our products. Your opinions areanswer some questions about your experience buying and using our products. Your opinions are
important to us. You are of course always welcome to contact us via your BK Medical representative orimportant to us. You are of course always welcome to contact us via your BK Medical representative or
by contacby contac
ting us directing us direc
tlytly
..
If you have comments about the user documentation, please write to us at the email address above. WeIf you have comments about the user documentation, please write to us at the email address above. We
would like to hear from you.would like to hear from you.
System SoftwareSystem Software
••
NOT FAULT TOLERANTNOT FAULT TOLERANT
. THE SOFTWARE IS NOT FAULT TOLERANT. BK Medical HAS. THE SOFTWARE IS NOT FAULT TOLERANT. BK Medical HAS
INDEPENDENTLY DETERMINED HOW TO USE THE SOFTWARE IN THE DEVICE, AND MS HASINDEPENDENTLY DETERMINED HOW TO USE THE SOFTWARE IN THE DEVICE, AND MS HAS
RELIED UPON BK Medical TO CONDUCT SUFFICIENT TESTING TO DETERMINE THAT THERELIED UPON BK Medical TO CONDUCT SUFFICIENT TESTING TO DETERMINE THAT THE
SOFTWARE IS SUITABLE FOR USE.SOFTWARE IS SUITABLE FOR USE.
••
EXPORT RESTRICTIONSEXPORT RESTRICTIONS
. You acknowledge that Windows 8 Embedded is . You acknowledge that Windows 8 Embedded is
of US-origin. You agree to complyof US-origin. You agree to comply
with all applicable international and with all applicable international and
national laws that apply to Windows 8 Embedded, including the U.S. Exportnational laws that apply to Windows 8 Embedded, including the U.S. Export
Administration Regulations, as well as end-user, end-use and country destination restrictions issued by U.S. andAdministration Regulations, as well as end-user, end-use and country destination restrictions issued by U.S. and
other governments. For additional information on exporting Windows 8 Embedded, see http://other governments. For additional information on exporting Windows 8 Embedded, see http://
www.microsoft.com/exporting/www.microsoft.com/exporting/
••
The bk3000 Ultrasound System is closed. Any modification of or installation of software to the system mayThe bk3000 Ultrasound System is closed. Any modification of or installation of software to the system may
compromise safety and function of the system. Any modification of or installation of software without writtencompromise safety and function of the system. Any modification of or installation of software without written
permission from permission from
BK BK
Medical wMedical w
ill immill imm
ediately void ediately void
any warrantany warrant
y supplied y supplied
by BK by BK
Medical. Such Medical. Such
changes will changes will
alsoalso
void any service contract and result in charges to the customer for restoration of the original bk3000 Ultrasoundvoid any service contract and result in charges to the customer for restoration of the original bk3000 Ultrasound
System.System.
Trademarks:Trademarks:
DICOM is the registered trademark of the DICOM is the registered trademark of the
National Electrical Manufacturers Association for its standards National Electrical Manufacturers Association for its standards
publicationspublications
relating to digital communications of medical information.relating to digital communications of medical information.
Microsoft and Windows are registered trademarks of Microsoft Corporation in the United States and other countries.Microsoft and Windows are registered trademarks of Microsoft Corporation in the United States and other countries.
bk3000 = Ref. Type 2300bk3000 = Ref. Type 2300
© 2015 BK Medical© 2015 BK Medical
Information in this document may be subject to change without notice.Information in this document may be subject to change without notice.

33
ContentsContents
Chapter Chapter
1 1
General General
Information . . Information . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . 5. . 5
Essential PeEssential Pe
rformancerformance
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . .
55
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Modes of Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6Modes of Operation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
IndicatioIndicatio
ns for Use ns for Use
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . .
77
Contraindications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7Contraindications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Chapter Chapter
2 2
Safety Information Safety Information
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. 9. 9
Safety Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9Safety Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Safety SymbSafety Symb
ols and Information on the Eqols and Information on the Eq
uipment . . . . . . uipment . . . . . .
. . . . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. .
99
General Safety Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10General Safety Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
MechanMechan
ical Safetyical Safety
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
1212
Explosion Hazards. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12Explosion Hazards. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Electrical Safety. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13Electrical Safety. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
ESD Training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13ESD Training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Electrical Noise. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13Electrical Noise. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
RF (Radio Frequency) Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14RF (Radio Frequency) Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
InstallationInstallation
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
1515
ConnectinConnectin
g Other Equg Other Equ
ipment ipment
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . .
1515
Network Network
ConnectioConnectio
n . . . . . . . . . . . n . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . 16. . . . . . 16
Network Network
Security . . . . . . . . . . Security . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . 16. . . . . . 16
Network Network
Printing Printing
. . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . 16. . 16
Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
EMC RequirEMC Requir
ements ements
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . .
2020
Isolation of DICOIsolation of DICO
M Network. . . . . . . . . M Network. . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . .
2121
Wireless Networks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21Wireless Networks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Medical Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23Medical Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Non-MedNon-Med
ical Equipical Equip
ment ment
. . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
. . 24. . 24
Computer Security. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24Computer Security. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Service and Repair. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25Service and Repair. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Transducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25Transducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
During an Examination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25During an Examination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Checking thChecking th
e Date e Date
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . .
2525
Verifying the TrVerifying the Tr
ansducer Typansducer Typ
e. . . . . e. . . . .
. . . . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . .
2626
Measurements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26Measurements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
VFI - Vector Flow Imaging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27VFI - Vector Flow Imaging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Puncture anPuncture an
d Brachythed Brachythe
rapy. . . . . rapy. . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . . . .
. . . . . . . . . . . .
. . . . . . . . . .
2727
3D3D
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2828
Picture in PiPicture in Pi
cture cture
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . .
2929
Acoustic Output. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29Acoustic Output. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Monitor Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30Monitor Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Thermal and MThermal and M
echanical Indechanical Ind
ices . . ices . .
. . . . . . . . . . . .
. . . . . . . . . . . .
. . . . . . . . . . . .
. . . . . . . . . . . .
. . . . . . . . . .
. . . .
3030

44
Acoustic OuAcoustic Ou
tput Meastput Meas
urementurement
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . .
3131
FunctionFunction
s Affecting Acouss Affecting Acous
tic Output. . . . tic Output. . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. .
3232
Default Acoustic Output. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32Default Acoustic Output. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
Clinical MeasuClinical Measu
rements: Ranges anrements: Ranges an
d Accuracies. . . . . d Accuracies. . . . .
. . . . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . . . .
. . . . . .
3333
Geometric Measurements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34Geometric Measurements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Time MeasuTime Measu
rementsrements
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . .
3535
Doppler Measurements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35Doppler Measurements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Chapter Chapter
3 3
Getting Getting
Started Started
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
..
3737
Index Index
. . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. .
3939
Appendix Appendix
A A
Warnings Warnings
and and
Cautions Cautions
Displayed Displayed
on on
the the
System . System .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
4343
English source versionEnglish source version
16-01249-EN-0116-01249-EN-01

bk3000 User Guidebk3000 User Guide
((
11
66
--
00
11
22
44
99
--
EE
NN
--
00
11
))
GG
ee
nn
ee
rr
aa
l l
II
nn
ff
oo
rr
mm
aa
tt
ii
oo
nn
55
Chapter 1Chapter 1
General InformationGeneral Information
This user guide is for the bk3000 ultrasound system.This user guide is for the bk3000 ultrasound system.
NOTE:NOTE:
Some of the functionality and options described in this guide may not beSome of the functionality and options described in this guide may not be
available with your version of the system.available with your version of the system.
Before using the equipment, please make yourself familiar with the information inBefore using the equipment, please make yourself familiar with the information in
the accompanying user information documents. Some documents are printed. Makethe accompanying user information documents. Some documents are printed. Make
sure that you also read the transducer user guide and specifications for eachsure that you also read the transducer user guide and specifications for each
transducer that you use.transducer that you use.
TT
ableable
1-1.1-1.
User infoUser info
rmatiormatio
n documenn documen
tation thatation tha
t accompat accompa
nies the equipnies the equip
ment.ment.
Improper useImproper use
Failure to follow safety instructions or use for purposes other than those described inFailure to follow safety instructions or use for purposes other than those described in
the user manuals constitutes improper use.the user manuals constitutes improper use.
Essential PerformanceEssential Performance
The system can provide 2D and 3D ultrasound echo and flow imaging systems as anThe system can provide 2D and 3D ultrasound echo and flow imaging systems as an
aid in diagnosis, data processing and -transfer, and guidance of puncture and biopsy.aid in diagnosis, data processing and -transfer, and guidance of puncture and biopsy.
The system can perform simple geometric measurements and calculations.The system can perform simple geometric measurements and calculations.
The system can guide biopsy- and puncture needles.The system can guide biopsy- and puncture needles.
The system is free from artefacts or distortion in the image or error The system is free from artefacts or distortion in the image or error
of a displayedof a displayed
value, which can be attributed to a physiological effect and which may alter thevalue, which can be attributed to a physiological effect and which may alter the
diagnosis.diagnosis.
DD
oo
cc
uu
mm
ee
nn
tt
II
nn
ff
oo
rr
mm
aa
tt
ii
oo
nn
SS
yy
ss
tt
ee
m m
UU
ss
ee
r Gr G
uu
ii
dd
ee
II
nn
tt
rr
oo
dd
uu
cc
tt
oo
rr
y y
ii
nn
ff
oo
rr
mm
aa
tt
ii
oo
nn
, ,
ss
aa
ff
ee
tt
y y
ii
nn
ff
oo
rr
mm
aa
tt
ii
oo
nn
, ,
gg
ee
tt
tt
ii
nn
g sg s
tt
aa
rr
tt
ee
dd
..
Getting Getting
Started Started
User User
interface, interface,
basic basic
operating operating
instructionsinstructions
. .
Note: Note:
this this
book book
is is
part part
of of
thethe
system user guide.system user guide.
SySy
stst
em em
AdAd
vava
ncnc
ed ed
UsUs
er er
GuGu
idid
ee
InIn
fofo
rmrm
atat
ioio
n n
abab
ouou
t t
adad
vava
ncnc
ed ed
fufu
nctnct
ioio
nsns
, ,
glgl
osos
sasa
ryry
..
PP
rr
oo
dd
ucuc
t Dat Da
ta fta f
oo
r syr sy
stst
emem
SpSp
ecec
ii
fifi
cc
aa
titi
oo
ns ns
ff
oo
r tr t
he he
sysy
stst
emem
, i, i
ncnc
lulu
dd
inin
g dg d
isis
inin
ff
ecec
titi
oo
n mn m
ee
thth
oo
dd
s ts t
hh
aa
t ct c
an an
bb
ee
used. Indications for use for each transducer that can be used with theused. Indications for use for each transducer that can be used with the
system.system.
TT
ee
chch
nini
caca
l Dl D
aa
ta ta
((
BZBZ
22
1010
0)0)
AA
cc
oo
uu
stst
ii
c oc o
utut
pupu
t dt d
aa
tt
aa
, c, c
lili
nini
caca
l ml m
eaea
ss
uu
rr
ee
meme
nn
ts ts
((
rara
nn
gege
s as a
nd nd
aa
cc
cucu
rr
aa
cici
eses
),),
factory default power levels and data about EMC (electromagneticfactory default power levels and data about EMC (electromagnetic
compatibility) for all transducers. Pro Package calculation formulas.compatibility) for all transducers. Pro Package calculation formulas.
CC
aa
rr
e e
aa
nn
d d
CC
ll
ee
aa
nn
ii
nn
gg
CC
ll
ee
aa
nn
ii
nn
gg
, ,
dd
ii
ss
ii
nn
ff
ee
cc
tt
ii
oo
nn
, ,
ss
tt
ee
rr
ii
ll
ii
zz
aa
tt
ii
oo
nn
, ,
cc
hh
ee
cc
kk
ii
nn
gg
, ,
ss
tt
oo
rr
ii
nn
g g
aa
nn
d d
dd
ii
ss
pp
oo
ss
ii
nn
g g
oo
ff
BK Medical equipment. Includes environmental limits.BK Medical equipment. Includes environmental limits.
TT
rr
aa
nn
ss
dd
uu
cc
ee
r Ur U
ss
ee
r Gr G
uu
ii
dd
ee
SS
pp
ee
cc
ii
ff
ii
c ic i
nn
ss
tt
rr
uu
cc
tt
ii
oo
nn
s fs f
oo
r tr t
hh
e te t
rr
aa
nn
ss
dd
uu
cc
ee
r ar a
nn
d pd p
uu
nn
ctct
uu
rr
e ae a
tt
tt
aa
cc
hh
mm
ee
nn
tt
ss
..
ProPro
duct Dduct D
ata fata f
or eaor ea
ch trach tra
nsdunsdu
cercer
SpeciSpeci
ficafica
tiontion
s for ts for t
he trahe tra
nsdunsdu
cercer
, incl, incl
udinudin
g disig disi
nfecnfec
tion mtion m
ethoetho
ds thads tha
tt
can be used.can be used.

6
Chapter
1
January
2015
bk3000 User Guide
(16-01249-EN-01)
The system displays correct numerical values associated with the diagnosis to be
performed
.
The As Low As Reasonably Practicable (ALARP) principle is used and safety
related indications (MI, TIS, TIB, etc) are displayed as worst-case values.
The system does not generate unintended or excessive ultrasound output or
transducer surface temperature.
There is no unintended or uncontrolled motion of transducer assemblies intended for
intra-corpor
eal
use.
Intended Use
The system is intended for diagnostic ultrasound imaging or fluid flow analysis of
the human body, data processing and guidance of puncture and biopsy.
The system performs simple geometric measurements and calculations in the
following areas:
•
Urology
•
Vascular
•
Cardiology
•
OB/GYN
Modes of Operation
•
B-Mode (including Tissue Harmonic imaging)
•
M-Mode
•
PWD Mode
•
CFM Mode
•
Power Doppler
•
Contrast Imaging
1
•
CW Doppler
2
•
Elastography
3
1. In the USA, contrast-enhanced ultrasound has not been market cleared by the FDA, with the
exception of select cardiac imaging applications.
2. CW Doppler on the bk3000 has not been market cleared by the FDA or licensed by Health
Canada.
3. Elastography on the bk3000 has not been market cleared by the FDA or licensed by Health
Canada.

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
G
e
n
e
r
a
l
I
n
f
o
r
m
a
t
i
o
n
7
Indications for Use
The system is intended for use by qualified physicians for ultrasound evaluation.
Specific clinical applications and exam types include:
•
Fetal (including Obstetrics)
•
Abdominal
•
Pediatric
•
Small Organ (also known as Small Parts)
•
Adult Cephalic (also known as Adult Transcranial)
•
Neonatal Cep
halic
•
Intraoperative*
•
Intraoperative (Neuro)*
•
Transrectal
•
Transvaginal
•
Transurethral*
•
Musculoskeletal (Conventional and Superficial)
•
Cardiac Adult
•
Peripheral Vessel (also known as Peripheral Vascular)
Indicated uses are different for different transducers. The Product Data sheet for the
system contains a table listing the indicated uses for each transducer that can be used
with the system.
Contraindications
•
The bk3000 ultrasound system is not intended for ophthalmic use or any use
causing the acoustic beam to pass through the eye.
•
The Cardiac Adult application is not intended for direct use on the heart.
* This application currently not for sale

8
Chapter
1
January
2015
bk3000 User Guide
(16-01249-EN-01)

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
S
a
f
e
t
y
I
n
f
o
r
m
a
t
i
o
n
9
Chapter 2
Safety Information
The system can be used
for continuous operation, but imaging duration for individual
patients
must
not ex
ceed
60 m
inutes. W
e reco
mmend,
however
, that
you tu
rn of
f the
system at the end of each workday.
Safety In
formatio
n
This user guide contains cautions, warnings and other information about what you
must do to ensure the safe and proper performance of the ultrasound system. You
must also follow local government rules and guidelines at all
times.
NOTE:
Notes contain information that you should b
e aware of.
Safety Symbols and Information on the
Equipment
Table 2-1
contains brief explanations of the symbols and i
nformation used to label
the equipment. (Some labels in the table may appear on the transducer.)
BK Medical disclaims all responsibility for the operating safety, reliability and
performan
ce of
the equ
ipment
if these
symbols
and wa
rnings a
re disreg
arded
in any
way.
WARNING
Warnings contain information that is important for avoiding personal injury.
Caution
Cautions contain information and instructions that must be followed to avoid
damaging equipment, data, or software.
S
y
m
b
o
l
N
a
m
e
D
e
s
c
r
i
p
t
i
o
n
Cau
tion
or W
arni
ng
Con
sult
acc
ompa
nyin
g use
r gu
ides
when
you
encounter this sign on the instrument, to avoid
reducing its safety.
Consult instructions
for use
Consult user guide or other instructions.
Follow instructions
for use
Consult user guide or other instructions.
Push
ing p
roh
ibite
d
Do n
ot u
se e
xce
ssiv
e fo
rce
to p
ush t
he sy
stem
.
Excessive force when pushing over uneven surfaces
can cause the system to overbalance and tip.
T
able
2-1.
Symbo
ls and in
formati
on on th
e equi
pment
.

10
Chapter
2
January
2015
bk3000 User Guide
(16-01249-EN-01)
General Safety Precautions
The ultrasound system is designed and tested in accordance with EN/IEC 60601-1
(2006) (Part 1: General requirements for basic safety and essential performance) and
EN 60601–2–37 (2007) (Particular requirements for the basic safety and essential
performan
ce of ultraso
nic medica
l diagnostic
and mon
itoring equip
ment).
Ke
ep h
an
ds c
le
ar
Sh
ow c
au
ti
on w
he
n yo
u ad
ju
st t
he s
ys
te
m mo
ni
to
r
.
M
a
n
u
f
a
c
t
u
r
e
r
L
e
g
a
l
m
a
n
u
f
a
c
t
u
r
e
r
.
UL Classification for
Canada and US
UL requirements are met for special conditions.
Rx only
Federal law in North America restricts this device to
sale to, or on the order of, a physician.
Potential
Equalization
Terminal connected to the chassis. Should be
connected to corresponding terminals on other
equipment to eliminate potential differences.
G
r
ou
nd
(e
a
rt
h)
A
d
d
it
io
n
al
p
r
o
te
ct
i
v
e g
r
o
u
nd
(
ea
rt
h
).
T
y
p
e
B
F
B
F
:
I
s
o
l
a
t
e
d
f
r
o
m
g
r
o
u
n
d
.
Maximum patient leakage current under
•
Normal condition
100
A
•
Single-fault
condition
500
A
T
y
p
e
B
F
B
F
,
d
e
f
i
b
r
i
l
l
a
t
o
r
-
p
r
o
o
f
T
y
p
e
B
B
:
M
a
x
i
m
u
m
p
a
t
i
e
n
t
l
e
a
k
a
g
e
c
u
r
r
e
n
t
u
n
d
e
r
•
Normal condition
100
A
•
Single-fault
condition
500
A
S
e
a
l
i
n
g
D
u
s
t
- a
n
d
i
m
m
e
r
s
i
o
n
-
p
r
o
t
e
c
t
e
d
a
c
c
o
r
d
i
n
g t
o
E
N
60529.
S
t
a
n
d
b
y
S
y
m
b
o
l
o
n
O
N
/
S
t
a
n
d
b
y
b
u
t
t
o
n
o
n
b
a
c
k
o
f
s
c
a
n
n
e
r
unit – used to turn system on and off.
ESD
(electrosta
tic
discharge)
Do not touch pins in connectors with this symbol
unless you follow ESD precautionary procedures.
W
E
E
E w
a
s
t
e
W
i
t
h
i
n t
h
e
E
U
,
w
h
e
n
y
o
u
d
i
s
c
a
r
d
t
h
e e
q
u
i
p
m
e
n
t
, y
o
u
must send it to appropriate facilities for recovery and
recycling.
China ROHS 25 Years
Lifetime
Environmentally Friendly Use Period for ROHS is 25
years.
S
y
m
b
o
l
N
a
m
e
D
e
s
c
r
i
p
t
i
o
n
T
able
2-1.
Symbo
ls and inform
ation on the e
quipm
ent. (co
ntinue
d)
3D56

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
S
a
f
e
t
y
I
n
f
o
r
m
a
t
i
o
n
11
The system also complies with ANSI/AAMI ES60601-1 (2005) and CAN/CSA
C22.2 No.601.1 (2008).
It fulfills the
requiremen
ts for dust
protection (IP20) for ordinary equipmen
t
specified in EN 60529.
Physicians
only
Caution
Rx-c1
Federal law in North America restricts this device to sale to, or on the order of, a
physician.
Proper
Training
WARNING
GS-w1
Before you attempt to use BK Medical equipment, you should be trained in
ultrasonography or be under the supervision of someone who is trained in
ultrasonography. You should also be thoroughly familiar with the safe operation of your
ultrasound system: read all the user documentation that accompanies it.
No further training is required, but BK Medical offers training in how to use the system.
Consult your BK Medical representative for information.
Equipment
failure
WARNING
GS-w2
If at any time the system malfunctions, or the image is severely distorted or degraded, or
you suspect in any way that the system is not functioning correctly:
•
Remove all transducers from contact with the patient.
•
Turn off the system. Unplug the system from the power source.
•
Do not try to repair the system yourself.
•
Contact your BK Medical representative or hospital technician.
Isolating the
system
WARNING
GS-w3
The power supply cord connects the equipment to the line voltage. To isolate the
equipment, you must unplug the power supply cord from the power source. Do this
before you try to make any repairs to the system.
Spilled
liquids
Caution
S-c2
Do not spill liquids on the equipment.
Conden-
sation
Caution
S-c3
Large variations in temperature or humidity may cause water to condense inside the
system. If this happen
s, the system may fail to operate properly. Always let the system
come to room temperature before you plug it in.
•
Wait at least 2 hours after the system has been subjected to major changes in
temperature or humidity.
•
If there is visible evidence of condensation, wait at least 8 hours.

12
Chapter
2
January
2015
bk3000 User Guide
(16-01249-EN-01)
Before you use the equipment, make sure that all the safety requirements described
in this chapter have been satisfied.
Mechanical Safety
Mechanical failure or unintended use of ultrasound equipment can result in physical
injury to patients or
operators.
Explosion Hazards
Mechanical
injury
WARNING
MS-w1
Be careful to avoid the following potential sources of injury:
•
Parts of the body can be pinched by moveable parts of the equipment, such as the
control panel.
•
Tilting the system can cause it to be unstable and injure someone.
Do not lean or sit on the control panel or any other part of the system. The control panel
or monitor can break if subjected to heavy weights or impact.
All parts
must be
stable
WARNING
MS-w2
When parts of the equipment can be mounted individually (for example, for use in an
operating room) each part must be securely mounted to a stable support so that it does
not tip, fall or come loose and injure someone.
Don’t drop
the scanner
unit
WARNING
MS-w3
To avoid personal injury or damage to the system, if you handle the scanner unit by itself,
make sure you have a firm grip so that you do not drop it. Note that it may be hot.
Don’t push
too hard
WARNING
MS-w4
To
avoid injury and equipme
nt damage, do not push the system too hard, especi
ally when
you roll the system over an uneven surface. Applying excessive force near the top could
cause the system to overbalance and tilt.
Explosion
hazards
WARNING
EH-w1
The equipment is not designed to be used in potentiall
y explosive environments. It should
not be operated in the presence of flammable liquids or gases, or in oxygen-enriched
atmospheres.
There is a possible explosion hazard if the equipment is used in the presence of flammable
anesthetic. The system shou
ld be placed at least 25
cm (10 inches) from the patient.
The ultrasound system contains a lithium battery. Never remove or replace this battery.
The lithium battery must not be removed except by a BK Medical service representative.

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
S
a
f
e
t
y
I
n
f
o
r
m
a
t
i
o
n
13
Electrical Safety
ESD Training
The ESD Symbol
Anyone using the equipment must be able to recognize the ESD symbol and
understand how to take the necessary precautionary procedures, as described in the
caution below.
Interference
The 2300 Ultrasound System is suitable for use in all establishments, other than
domestic establishments and those directly connected to the public low-voltage
power sup
ply network
that supplies
buildings u
sed for do
mestic purp
oses.
Electrical Noise
Do not use a
power strip
WARNING
ES-w1
Do not plug the equipment into an ordinary power strip. If the ground connection fails,
this is dangerous because
•
the total leakage current for all the connected equipment can exceed the limits
specified in
EN/IEC
60601-1
(Part 1: General requirements for safety).
•
the impedance of the ground connection could exceed the limits specified in EN/IEC
60601-1.
Electrical
shock
WARNING
ES-w3
You risk electrical shock if you try to get inside the equipment (other than opening a cover
to access connectors or batteries described in the user guide). Do not allow anyone but
qualified service personnel to service the
equipment
.
ESD
Caution
ESD-c1
Do
not
touch
pins
in
connectors
that
have
the
ESD
symbol
.
Do
not
connect
anything to them unless you follow these ESD (electrostatic discharge) precautionary
procedures:
•
Discharge your body to ground before you touch the pins with your hand or a tool. For
example, touch an unpainted metal part of the system cover.
•
You can use a wrist strap connected to the additional protective ground or potential
equalization terminal on the system if that is more convenient.
Electrical
noise
WARNING
EN-w1
Electrical noise from nearby devices such as electrosurgical devices – or from devices that
can transmit electrical noise to the AC line – may cause disturbances in ultrasound images.
This could increase the risk during diagnostic or interventional procedures.

14
Chapter
2
January
2015
bk3000 User Guide
(16-01249-EN-01)
Electromagnetic I
nterferenc
e
Medical electrical equipment requires special precautions regarding EMC
(electromagnetic compatibility). You must follow the instructions in this chapter
when you install the system and put it into service.
If the image is distorted, it may be
necessary to position the system further from
sources of electromagnetic interference or to install magnetic shielding.
EMC noise can reduce the usable image depth. Therefore, to avoid having to repeat
an ultrasound examination, you must make sure beforehand that the ultrasound
system can be used for the examination. Repeating an examination can be regarded
as a potential risk that should be avoided, especially if the examination involves
transducers used intracorporeally or transducers used for puncture.
RF (Radio
Freq
uency) Interference
Portable and mobile RF (radio frequency) communication equipment can affect the
system, but the system will remain safe and meet essential performance
requirements.
An ultrasound system intentionally receives RF electromagnetic energy for the
purpose
of its operatio
n. The trans
ducers are
very sen
sitive to frequ
encies within
their signal frequency rang
e (0.3
MHz to 80
MHz). Therefore RF equip
ment
operating in this frequency range can affect the ultrasound image. However, if
disturbances occur, they will appear as white lines in the ultrasound image and
cannot be confused with physiological signals.
Other
equipment
nearby
WARNING
EMC-w1
Do not use this equipment adjacent to other equipment. If you must place it next to or
stacked with other equipment, verify that it operates normally there and neither causes
nor is affected by electromagnetic interference.
Possible
interference
sources
Caution
Inter-c1
Other equipment may interfere with the system, even if that other equipment
complies with CISPR
(Internati
onal Special Committee on
Radio Interference)
emission requirements.
Use specified
equipment
only
Caution
Inter-c2
Caution:
If you use accessories, transducers or cables with the system, other than those
specified, increased emission or decreased immunity of the system may result.

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
S
a
f
e
t
y
I
n
f
o
r
m
a
t
i
o
n
15
Installation
Original
power cords
If the original power cords are missing or damaged, you must order new ones from
your local BK Medical representative.
Additional Protective Ground and Potential Equalization
An
additional
protective
ground
can
be
connected
to
the
terminal
underneath
the
control panel, see
Fig 2-1
.
The
potential
equalization
terminal
underneath
the
control
panel
is
connected
to
the system chassis. It can be connected to corresponding terminals on other
equipment to eliminate potentia
l differences. Do NOT use it for
additional protect
ive
grounding.
Figure
2-1. The terminals for
potential equalization
and additional protective ground
are underneath the control panel.
Connecting Other Equipment
For connection to other equipment, BK Medical systems have a communication
protocol on
top of TCP/I
P
Installation
safety
requirement
WARNING
I-w1
To ensure safe performance, a qualified electrician or hospital safety personnel must verify
that the equipment is correctly installed and that it complies with the following safety
requirements:
•
Use only the original power supply cord. In the USA, this is fitted with a hospital grade
three-prong grounded power plug. Never try to remove or change the plug on the
power supply cord.
•
All equipment must only be connected to a grounded AC power supply (or wall outlet)
that meets EN/IEC/NEC requirements or applicable local regulations. The examination
room’s grounding system should be checked regularly by a qualified electrician or
hospital safety personnel.
•
Never use extension cords. The increased lengt
h of the cord will increase the resistance
of the protective ground conductor and may increase the equip
ment’s leakage current
beyond an acceptable level.
•
Keep power cords, sockets and plugs clean and dry at all times.
•
Make sure that the power supply cord cannot be accidentally disconnected from the
power source or the equipment.

16
Chapter
2
January
2015
bk3000 User Guide
(16-01249-EN-01)
Network Connection
BK Medical’s range of ultrasound systems comply with the DICOM standard for
handling, storing, printing and transmitting information in medical
imaging.
DICOM includes a file format definition and a network communication protocol
which facilitates the exchange of data between electronic medical systems.
For detailed information about:
•
network requirements
•
network configuration
•
workflow between devices
•
technical specifications
•
safety
specification
s
see BK Medical’s DICOM conformance statement at
www.analogicultrasound.com/support/bk-medical/DICOM
Networ
k
Security
It is the responsibility of the on-site personnel or technician to maintain the IT-
network and identify, analyze, evaluate and control new risks caused by a change in
the network
configuratio
n.
If the applicable network connection does not meet the required characteristics of the
IT-network, the following hazardous situations may occur:
•
Corrupt patient data due to
network errors, see
W
arning Exam-w3
on page
26
•
System is unable to use the network due to faulty or overloaded network, see
W
arning GS-w1 on pa
ge
11
•
System overloads the network causing other equipment to fail.
Network
guidelines
NOTE:
If your system interacts with other equipment
directly or indirectly you must
ensure that your network is properly dimensioned and that critical equipment is
placed on
a s
eparate network.
Otherwise you
could ris
k overloading
the network
and
your equipment failing.
Network Printing
For printing on network printers, BK Medical supports protocols PCL 5, PCL 6 and
PS (Post Script).
Connectors
PC connectors for connecting the system to other equipment such as approved
printers an
d video e
quipment a
re located o
n the rear
of the syste
m. See
Fig 2-3
.
Connection
guidelines
WARNING
C-w1
Follo
w the
guidelines in EN
60601-1-1
(Safety requirements for medical electrical systems)
when you connect the system to other equipment.

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
S
a
f
e
t
y
I
n
f
o
r
m
a
t
i
o
n
17
Some connectors are used by the system. Do not use connectors that are not labelled
in
Fig 2-3
.
More information about the connectors is in
Table 2-2
. Information about the cables
to use is in
Table 2-4
.
The bk3000 ultrasound system has four transducer sockets on the side of the system.
See
Fig 2-2
.
Figure
2-2. Transducer sockets.

18
Chapter
2
January
2015
bk3000 User Guide
(16-01249-EN-01)
Figure
2-3.
System
connectors.
Power supply
cord goes here

bk3000 User Guide
(
1
6
-
0
1
2
4
9
-
E
N
-
0
1
)
S
a
f
e
t
y
I
n
f
o
r
m
a
t
i
o
n
19
T
abl
e
2-2
.
Sys
tem
con
nec
tor
s.
Video Output
Although 4 different video output signal formats are available, the image quality is
not the same for all of them.
DVI gives best
image quality
To get the best image quality possible, connect your monitor or other video
equipment using the output signal that gives the highest quality image. See the list
below
.
Output signal types (in order of quality, with digital DVI highest)
1
DVI - digital output that gives the best image quality.
2
VGA – this analog output from the DVI connector gives slightly poorer image
quality than the digital DVI
output.
3
S-video – analog output
4
Composite – signal with the most loss of information
If you must use a cable that does not have a DVI connector, you may need to use an
adapter.
T
able 2-
3
shows you which adapters can be used.
S
y
m
b
o
l
C
o
n
n
e
c
t
o
r
A
d
d
i
t
i
o
n
a
l
I
n
f
o
r
m
a
t
i
o
n
D
V
I
-
I
C
o
n
n
e
c
t
o
r
f
o
r
a
u
x
i
l
i
a
r
y
D
V
I
o
r
V
G
A
monitor.
Composite/S-
video
Out
7-pin
S-video
connector
(see
Table 2-3
for
pin layout) that can be adapted to a
composite video output
(see
Table 2-3
).
M
i
c
r
o
p
h
o
n
e
M
i
c
r
o
p
h
o
n
e
c
o
n
n
e
c
t
o
r
Audio In
Audio
Out
4
USB
2.0
connectors,
A-type
500mA
current
limit
on
each.
1
0
/
1
0
0
/
1
0
0
0 E
t
h
e
r
n
e
t
L
A
N
: 1
0
/
1
0
0
/
1
0
0
0 L
A
N c
o
n
n
e
c
t
o
r
, R
J
4
5
.
Table of contents
Other bk ultrasound Medical Equipment manuals