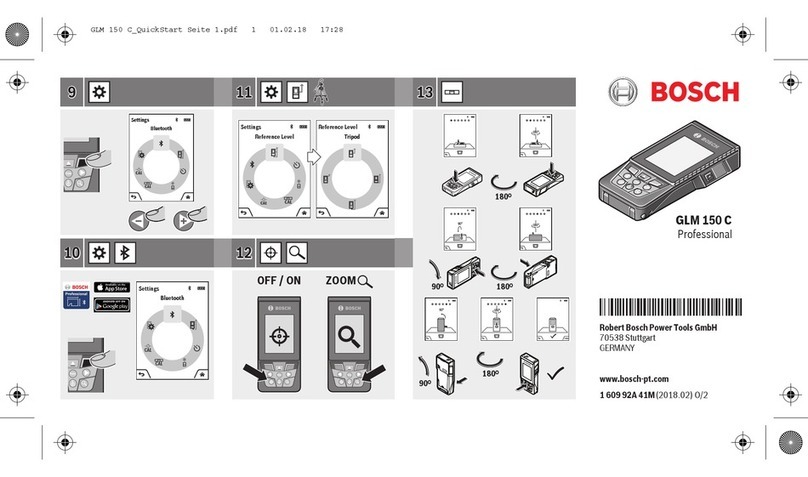
© Bosch Healthcare Solutions GmbH6Instructions for use
en
Your Vivatmo me
1.3 Intended use / Indications for use
Intended use: The Vivatmo me is intended for quantitative
measurement of fractional nitric oxide in human breath.
Indication: Measurement of changes in the fractional
nitric oxide concentration in expired breath aids in evalu-
ating a patient’s response to anti-inflammatory therapy,
as an adjunct to established clinical and laboratory as-
sessments of inflammatory processes such as asthma.
The Bosch Vivatmo me system is a non-invasive self-test-
ing device intended to be used at home (in-vitro diagnos-
tic use) as an aid to monitor airway diseases whilst under
the care of their physician or healthcare expert. Vivatmo
me measurement procedure requires patient’s coopera-
tion by breathing into the device via a disposable mouth-
piece. Patients should be 7 years or older and capable to
complete the breathing maneuver.
The measurement procedure of the Vivatmo me system
generates the fraction of the exhaled breath (FeNO) based
on the recommendations for the measurement of exhaled
respiratory nitric oxide of the European Respiratory Soci-
ety (ERS) and the American Thoracic Society (ATS). FeNO
is recommended by the ATS in the diagnosis of eosinophil-
ic airway inflammation and in determining the likelihood
of responsiveness to anti-inflammatory pharmacological
therapy in individuals with chronic respiratory symp-
toms possibly due to airway inflammation [ATS, 2011].
Vivatmo me should only be used as directed in the
Vivatmo me Instructions for use and as recommended by
healthcare professionals. Regardless of displayed test
results, if signs or symptoms of chest tightness, short-
ness of breath, coughing or wheezing occur, the user
should contact their healthcare professional immediately.
Contra-indications: Not known.