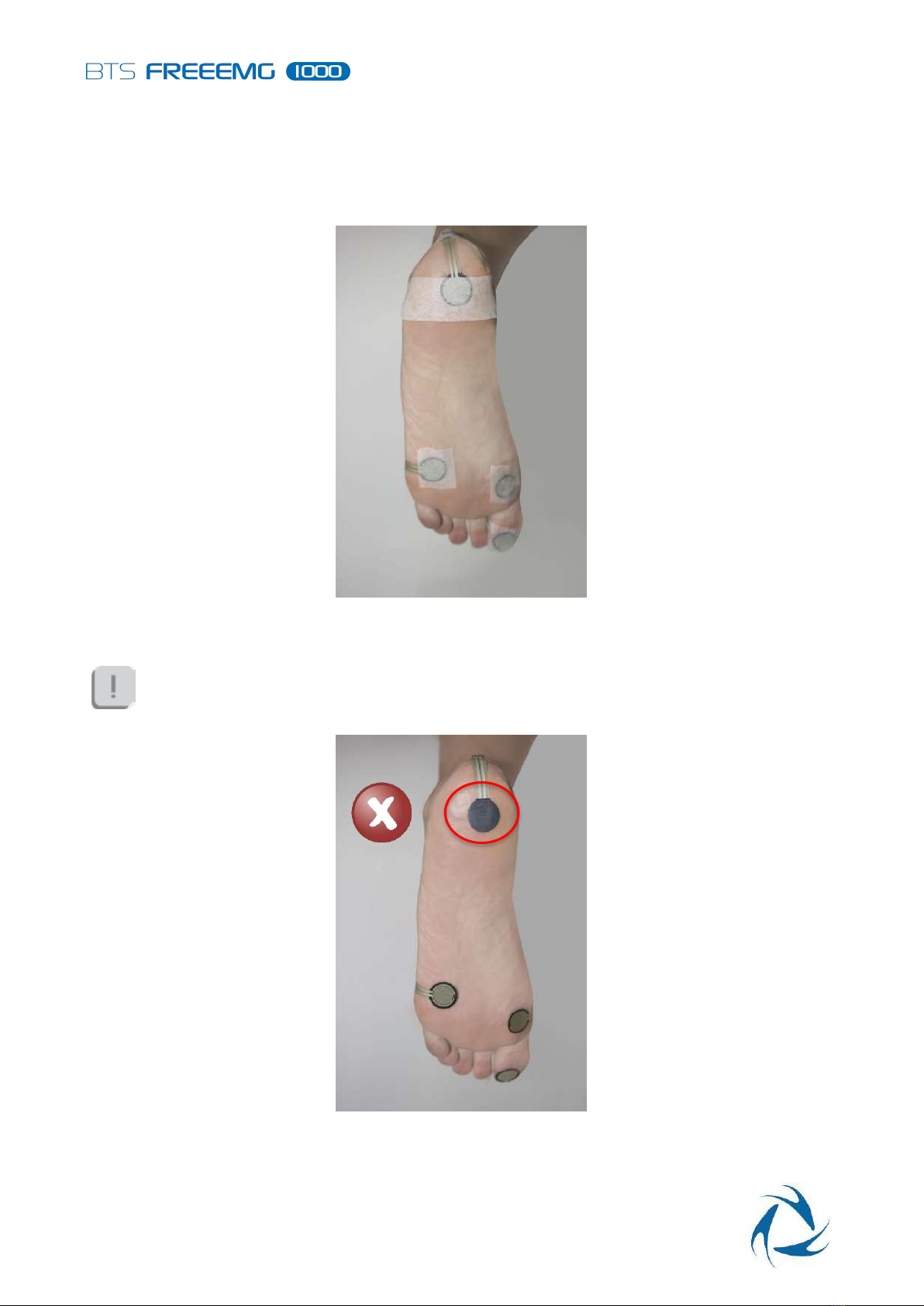
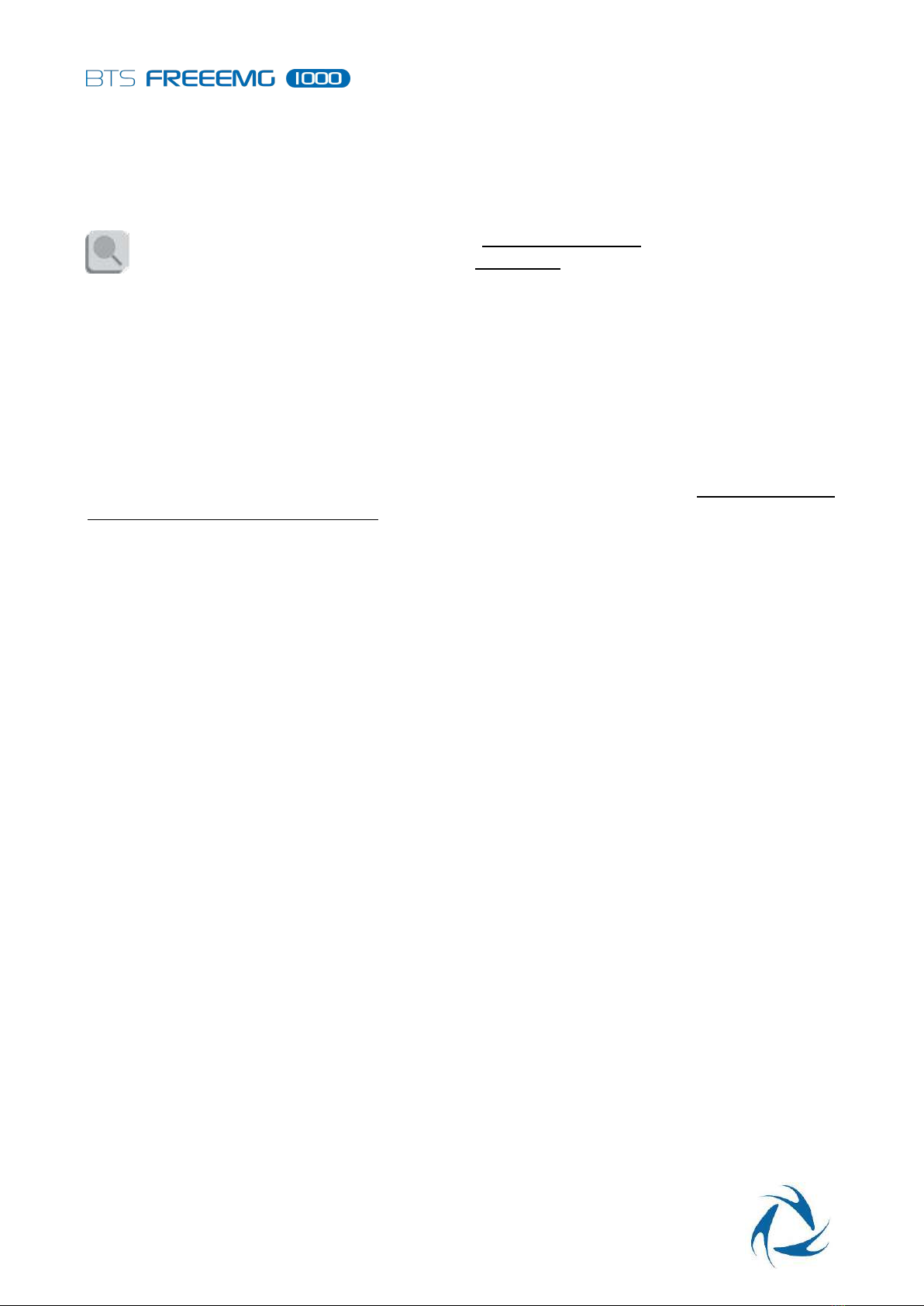
Warnings
83
13 Warnings
Safety information: Please read and follow these instructions carefully. The instrument's
safety cannot be guaranteed if they are not followed.
BTS FREEEMG 1000 is a medical device (EU Directive 93/42/EEC and its amendments, including
Directive 2007/47/EEC). Its use must be supervised at all times by qualified and authorized
personnel, according to the laws in force in the nation it is in use. The EMG probes are classified as
ETSI EN 300 440 “Receiver category 3” according to Directive R&TTE 99/5/EEC.
The results of the acquisitions must be assessed by people legally authorised by national laws, who
possess specific knowledge and competence on the anatomy and physiology of the muscular
system.
Since it has a high level of sensitivity (measured voltage levels of between 1 μV and 6 mV), the
instrument must be used in a typical commercial or hospital environment.
All pertinent state, regional, and local regulations for accident prevention, occupational safety, and
environmental protection must be observed when installing and using this product.
Do not wet or wash any electronic device component with water or other liquids. To clean the
system components use a soft cloth damped with neutral soap. System components are NOT
protected from liquid infiltration.
No modification of the equipment is allowed:
•Maintenance and repair of the BTS FREEEMG 1000 system (all components included) may
be carried out only by BTS S.p.A. authorized technicians.
•Modifying the product, substituting or changing any hardware or software part included in
the system could drastically affect performance and may void existing warranties.
•BTS S.p.A. cannot be held responsible for system safety in case of any alteration of the
product.
Under no circumstances the integrity of the system and of all its components must be compromised.
In case of malfunctioning or accidental damage of any system component, always address the
technical service for assistance.
See Appendix E –Technical Service and Repair for detailed information on how to contact
BTS Customer Service.
Use only the power supply unit FW7363M/09 (FRIWO) or an equivalent one provided by BTS S.p.A.
for supplying the charger unit. If a different power supply unit not expressly approved by BTS S.p.A.
is used, the compliance to IEC 60601-1 is not ensured. Moreover, the use of other power supply
units can damage the system.