ChemoMetec NucleoCounter NC-202 Technical manual

997-0005
NucleoCounter® NC-202™
Document Compilation
V 1.0
Page 1 of 127

Table of Contents
Introductory material Master Page No.
880-0028-76 Statement of excellence 4
Documents regarding the NC-202™ instrument
994-2030 Tech Note NucleoCounter® NC-202™ Performance data 5
994-0216 Effects of sample concentration on cell counting variation – NC-202™ 11
Certificate of Conformity – NC-202 14
Statement: Proprietary Article Certificate - NucleoCounter NC-200 15
Documents regarding consumables for the NC-202™ instrument
Statement: TSE BSE Statement 16
992-3101 Package Insert for Via2-Cassettes 17
Statement: ChemoMetec Cassette Conformity Statement 18
Certificate Example: 992-3102 Certificate of Analysis: Via2-Cassette 19
880-0030-76A QA Measures in the Production of ChemoMetec Cassettes 20
992-0041 Package Insert - 910-0010 Lysis 1 27
995-0078 SDS Solution 10 (UK) 28
Certificate Example: Certificate of Product Testing_910-0010_Lysis 1 32
992-2021 Package Insert - 912-2021 NC-202 IQ/OQ Kit 33
995-0072 SDS ChemoMetec Test Kits (UK) 34
Certificate Example: Certificate of Product Testing 912-2021_NC-202_IQ/OQ_Kit 38
992-2023 Package Insert - 912-2022 NC-202™ PQ Kit 39
995-0072 SDS ChemoMetec Test Kits (UK) 40
Certificate Example: Certificate of Analysis 912-2022 NC-202™ PQ Kit 44
Page 2 of 127

Documents for End-User Validation
994-2022 App Note 2022 NC-202™ IQ/OQ/Zero count protocol 45
994-2023 Tech Note NC-202™ IQ/OQ (check list) 48
994-2024 App Note 2024 NC-202™ PQ 50
994-2025 Tech Note NC-202™ PQ (check list) 53
994-2026_App Note 2026 Count & Viability – Via2-Cassette™ 57
994-2028 App Note 2028 Lysis Count – Via2-Cassette™ 60
User’s Guides and White Papers
991-2020 NucleoCounter® NC-202™ Instrument User Guide 64
991-2022 NC-View™ Software User Guide 93
991-2021 NC-View™ ‘Secure Mode’ Guide 115
994-2031 Tech Note 2031 CSV-file output documentation 124
Page 3 of 127

Document no: 880-0028-76 v. 1
Date of issue: 2015-04-22
ChemoMetec A/S Statement of Excellence
ChemoMetec A/S is committed to developing and improving analytical measurement techniques within the fields of
particle counting and liquid chemical composition determination, and to become the supplier and partner of choice
for end-users in every application area. In pursuit of this, we are dedicated to the following points:
Commitment to meeting customer requirements and to continually strive to improve the services offered by
the company.
Achieving continual improvements of our operations and performance.
Working to create a positive environment and internal culture that will attract and retain the best people.
Establish objectives to improve communications of organizational direction and drive improvements.
___________________________
Martin Glensbjerg
COO
ChemoMetec A/S
Page 4 of 127

Doc. No.: 994-2029 v. 1.1 · Issue date: 03-Apr-2020
Page 1 of 6
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Technical Note No. 2029 Rev. 1.1
NucleoCounter® NC-202™Performance data
The NucleoCounter® NC-202™ is a high precision cell counter using the Via2-Cassette™for sample loading
and staining. The data in this document demonstrates the performance of the NucleoCounter® NC-202™
in comparison with manual cell counting.
Introduction
Cell density greatly impacts cell behavior in a broad
range of cell-based applications such as research
experiments, bioassays, and bioprocessing.
Precise and robust cell counting is critical to achieving
reproducibility in such applications. The following
document summarizes the performance of the
NucleoCounter® NC-202™in comparison with manual
cell counting using a generic counting chamber (the
Bürker-Türk) and trypan blue.
Background
The NucleoCounter® NC-202™ is a high precision cell
counter using low magnification fluorescence
microscopy and automated image analysis to identify
live and dead cells.
The Via2-Cassette™ combines cell sampling, staining,
and loading of the counting chamber into a single
workflow. Together, the NucleoCounter® NC-202™and
Via2-Cassette™ generate data with low inter- and intra-
operator variation. The NucleoCounter® NC-202™ can
count all mammalian cell types, including primary cells
and aggregated cells.
NC-View™, the accompanying NucleoCounter® NC-
202™ software, provides operational control and easy
validation of cell counts by displaying images and
results in an intuitive user interface. NC-View™ is
designed to maintain data integrity and is compatible
with the 21 CFR Part 11 guidelines.
This document summarizes a complete dataset where
a large panel of cell lines were counted with three
NucleoCounter® NC-202™ instruments in parallel with
manual counting.
Conclusion
The NucleoCounter® NC-202™ displays superior
performance to manual counting in terms of linearity,
and precision and has low instrument-instrument
variation.
Experimental setup
A panel of cell types (Appendix I) were counted using three different NucleoCounter® NC-202™
instruments. Cell counts were performed using the standard ‘Count & Viability’ protocol with Via2-
Cassettes™. Manual cell counting was done in parallel, to serve as a counting reference. Manual counts
were carried out in duplicates using 0.4% trypan blue and a Bürker-Türk counting chamber. The same
operator performed all the manual cell counts to minimize counting variation.
Page 5 of 127

Doc. No.: 994-2029 v. 1.1 · Issue date: 03-Apr-2020
Page 2 of 6
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Cell concentration range
To confirm the accuracy of the NucleoCounter® NC-202™ instrument in the entire counting range (5x104to
1x107cells/ml), cell counts were performed on cell samples with a wide range of concentrations. The average
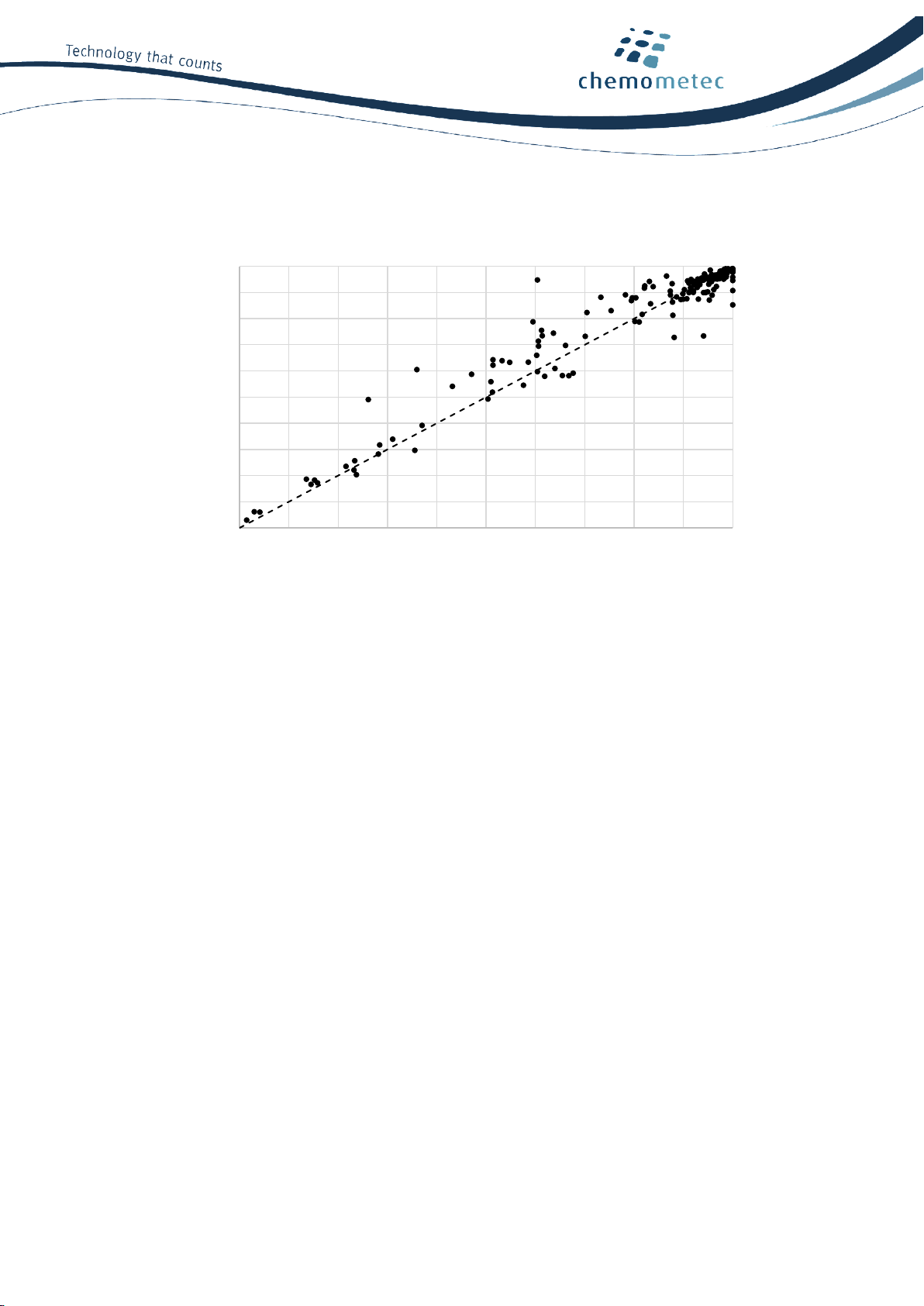
cell count value was plotted for the NucleoCounter® NC-202™and manual counts (Figure 1). These counts
showed a clear linear correlation with an R2of 0.918.
Figure 1. NucleoCounter®NC-202™ total cell count correlates with manual counting. The graph presents data from 15
different cell types with 166 measurements using three NucleoCounter®NC-202™ instruments and manual counting.
Viability range
The NucleoCounter® NC-202™ provides viability measurements from 0 –100% using the widely used stain
DAPI to quantify the number of non-viable cells. There is a clear linear correlation between viabilities
determined by the NucleoCounter® NC-202™ and by trypan blue exclusion in manual counting (Figure 2).
y = 1,0363x
R² = 0,918
1,E+04
1,E+05
1,E+06
1,E+07
1,E+04 1,E+05 1,E+06 1,E+07
NucleoCounter®NC-202™
Total cells/ml
Manual counting
Page 6 of 127
Doc. No.: 994-2029 v. 1.1 · Issue date: 03-Apr-2020
Page 3 of 6
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Figure 2. NucleoCounter®NC-202™ viability correlates with manual assessment by trypan blue. The graph presents
data from 15 different cell types with 166 measurements using three NucleoCounter®NC-202™ instruments and
manual counting.
Cell counting precision
The precision of a cell count depends on the number of cells counted. The variation of a cell count is assumed
to follow the Poisson probability distribution, where measurements of discrete events will deviate with the
square root of the number of events counted. In addition, variation in sample collection and processing will
also contribute to the overall deviation.
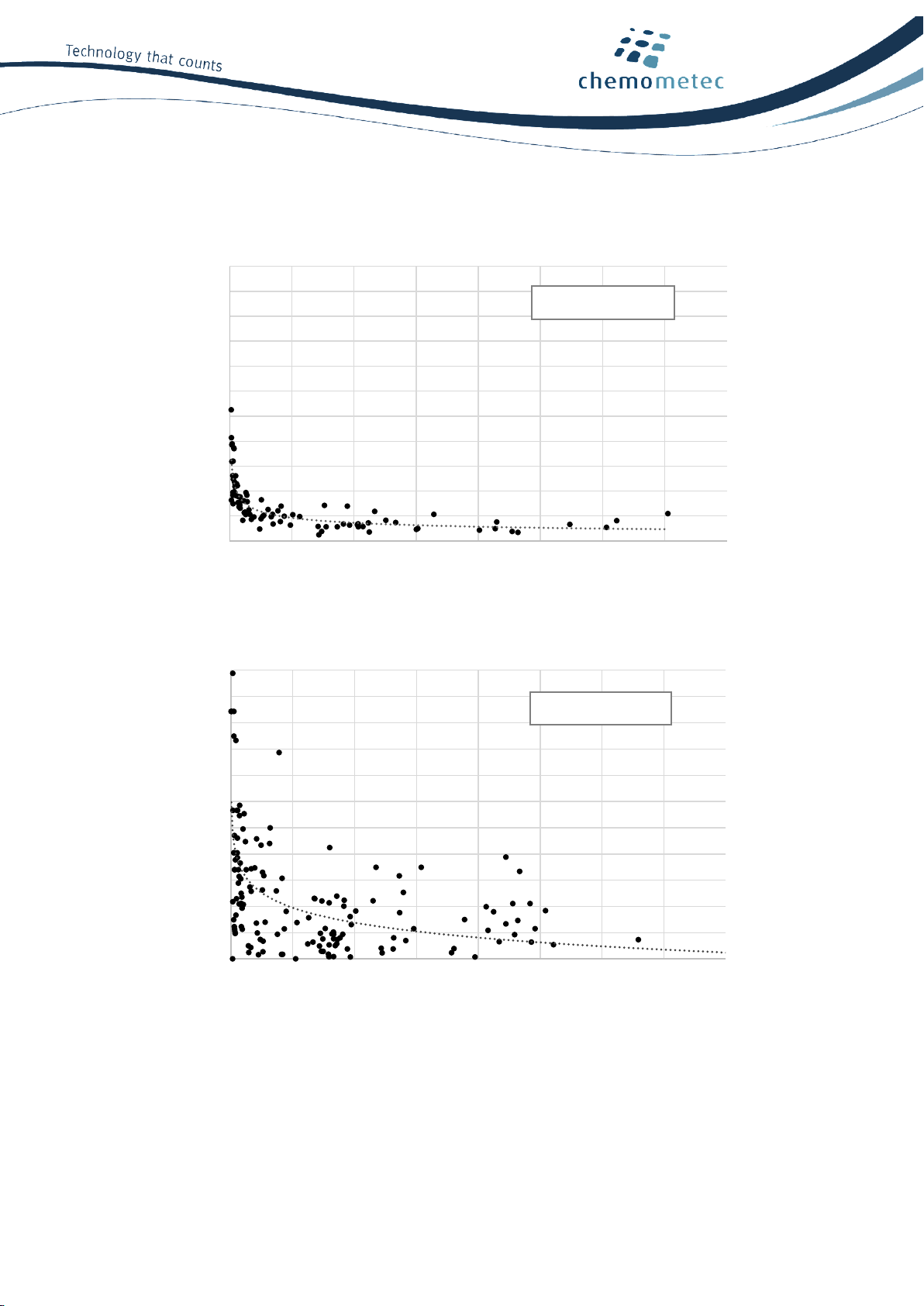
To demonstrate counting precision, the coefficient of variation (CV) was calculated from replica
NucleoCounter® NC-202™or manual counts and plotted against the cell concentration (Figure 3). The
NucleoCounter NC-202™showed significantly lower variation; on average 4.1% (Figure 3A) as compared to
an average of 8.2% for manual counting (Figure 3B).
0
10
20
30
40
50
60
70
80
90
100
010 20 30 40 50 60 70 80 90 100
NucleoCounter® NC-202™
Manual counting
Viability (%)
Page 7 of 127
Doc. No.: 994-2029 v. 1.1 · Issue date: 03-Apr-2020
Page 4 of 6
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Figure 3. NucleoCounter®NC-202™ cell counting is more precise than manual counting. The graph presents data from
15 different cell types with 166 measurements using (A) three NucleoCounter®NC-202™ instruments and (B) manual
counting. CV indicates coefficient of variation.
0
5
10
15
20
25
30
35
40
45
50
55
1,E+03 1,E+06 2,E+06 3,E+06 4,E+06 5,E+06 6,E+06 7,E+06
CV %
Cells/mL
NucleoCounter® NC-202™
A
0
5
10
15
20
25
30
35
40
45
50
55
1,E+03 1,E+06 2,E+06 3,E+06 4,E+06 5,E+06 6,E+06 7,E+06
CV%
Cells/mL
Manual counting
B
Average CV = 4.1%
Average CV = 8.2%
Page 8 of 127
Doc. No.: 994-2029 v. 1.1 · Issue date: 03-Apr-2020
Page 5 of 6
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Instrument-to-instrument repeatability
All NucleoCounter® NC-202™ instruments are calibrated to a reference instrument at time of manufacture
to ensure that all instruments acquire consistent data and perform to the same high standard. The LED light
sources are constant over time, which ensure stable image acquisition regardless of the age of the
instrument. The optics are mechanically adjusted during production and cannot be changed, and do not
require any adjustment by the user. Consequently, all NucleoCounter® NC-202™ instruments are
inter-comparable, regardless of production year.
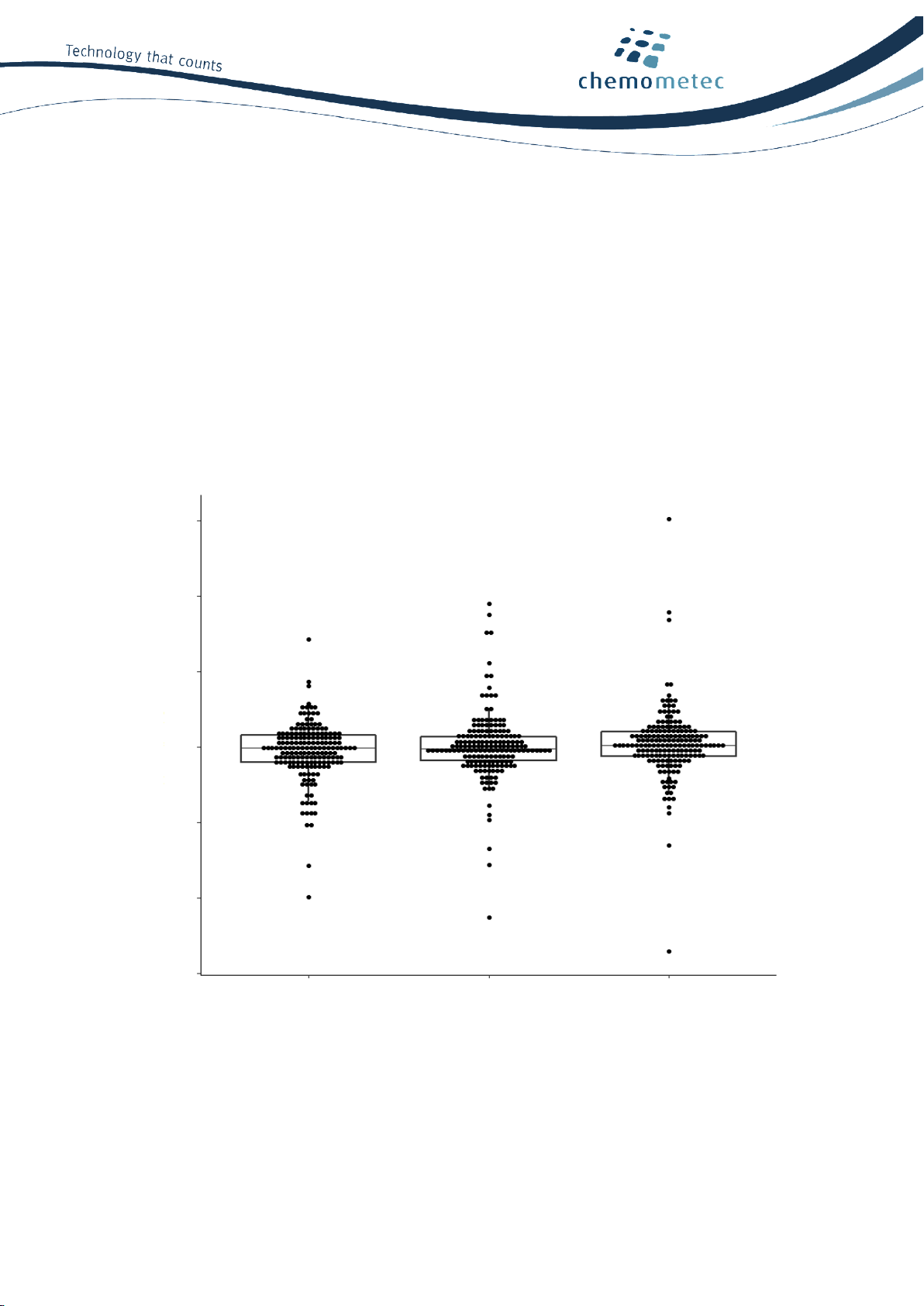
When normalized total cell counts are compared between three NucleoCounter® NC-202™ instruments, no
significant difference is observed, evident by a P-value of 0.998 in a one-way ANOVA test: n =228, 15
different cell lines (Figure 4).
Figure 4. Instrument-to-instrument variation. The graph presents normalized cell count data from 15 different cell
types at different concentrations (a total of 166 individual measurements) using three different NucleoCounter®NC-
202™ instruments. One-way ANOVA test shows no significant difference between the three NucleoCounter®NC-202™
instruments: P = 0.998.
Instrument
Normalized count
Count6
1,3
1,2
1,1
1,0
0,9
0,8
0,7
1
2
3
Page 9 of 127
Doc. No.: 994-2029 v. 1.1 · Issue date: 03-Apr-2020
Page 6 of 6
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Appendix I:
List of cell types used for this technical note.
Cell type
Species
Tissue
Remarks
3G5
Mouse
Blood
B lymphocyte, suspension
BSC-1
African Green Monkey
Kidney
Epithelial, adherent
BHK-21
Hamster
Kidney
Fibroblast, adherent
CHO
Chinese Hamster
Ovary
Epithelial-like, adherent
COS-7
African Green Monkey
Kidney
Fibroblast, adherent
ES-E14*
Mouse
Embryo
Embryonic stem cells, spherical, adherent
FreeStyle™ CHO-S
Chinese Hamster
Ovary
Epithelial-like, suspension
FreeStyle™ 293-F
Human
Embryonic Kidney
Epithelial, suspension
HEK293T
Human
Embryonic Kidney
Epithelial, adherent
HeLa
Human
Cervix
Epithelial, adherent
HMEC*
Human
Breast
Epithelial, adherent
JM1
Human
Blood
pre-B lymphoblast, suspension
Jurkat A3
Human
Blood
T lymphocyte, suspension
MCF7
Human
Mammary Gland
Epithelial, adherent
MDA-MB-231
Human
Mammary Gland
Epithelial, adherent
MR1
Mouse/Hamster
Blood
B lymphocyte, suspension
MSC*
Human
Adipose tissue
Stem cells
NIH/3T3
Mouse
Fibroblasts
Fibroblast, adherent
PBMC*
Human
Blood
Lymphocytes, suspension
PC-3
Human
Prostate
Epithelial, adherent
U-2 OS
Human
Bone
Epithelial, adherent
WEHI-S
Mouse
Fibrosarcoma
Epithelial, adherent
YAC-1
Mouse
Blood
Lymphoblast, suspension
*Primary cells
Page 10 of 127

Doc. No.: 994-2030 v. 1.0 · Issue date: 04-Apr-2020
Page 1 of 3
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
Technical Note No. 2030 Rev. 1.0
Effects of sample concentration on cell counting variation
NucleoCounter® NC-202™
This Tech Note explains the correlation between the number of cells counted and the precision of the
cell count. The purpose is to explain the reproducibility of differing cell counting methods based on the
number of cells counted.
Coefficient of Variation (CV) and Standard Deviation (SD)
Determining the precision of a cell culture’s concentration depends on the number of cells counted (n). The
counting of random events is normally assumed to follow a Poisson distribution (i.e. normal distribution),
according to which the standard deviation is the square root of the number of events (in this case, cells
counted). The relative precision, expressed as Coefficient of Variation (CV), is therefore:
To measure the variation of the ‘true’ count, CV is often preferred, as it can be expressed as a percentage
and allows for easy comparison of different sized samples.
The Standard Deviation (SD) is calculated as the square root of the number of cells counted. SD is expressed
in the same unit as the objects counted, in this case cells. For example, a sample with 400 cells has an SD of
20 cells (√400 = 20), written as 400 cells ± 20 cells. For the same sample, the expected CV following the
Poisson distribution is 5% (1/√400 × 100).
Cell
conc.
(cells/ml)
Counted
cells (n)
Poisson
SD (cells)
Poisson CV
(%)
5 × 104
68
8
12.2%
1 × 105
135
12
8.6%
5 × 105
677
26
3.8%
1 × 106
1353
37
2.7%
5 × 106
6765
82
1.2%
1 × 107
13530
116
0.9%
The Standard Deviation and Coefficient of
Variance (CV) expected solely from the Poisson
(i.e. normal) distribution of a population of cells
counted with the NucleoCounter® NC-202™.
Expected CV% derived from the Poisson distribution
against the number of cells counted (n) in the range
100 - 2000 n.
Page 11 of 127

Doc. No.: 994-2030 v. 1.0 · Issue date: 04-Apr-2020
Page 2 of 3
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
As both measures of variation are relative to the square root of the number of cells counted (n), the
relationship between the measures of variation from a ‘true’ count is not linear. In other words, the greater
the number of cells counted, the smaller the SD and CV. The following table gives examples of expected SD
and CV values at different cell counts and the graph explains the correlation between cell count and
expected SD.
Total standard deviation
The total standard deviation (SD) includes the contribution from Poisson along with several other sources
of variation:
SDtotal = √(SDPoisson2+ SDcounting device2+ SDhandling2+ SD…2)
Between these different sources of variation, the Poisson will be the greatest contributor. The SDcounting device
is very low due to the use of Via2-Cassette™ with the NucleoCounter® NC-202™. Every Via2-Cassette™ is
individually volume-calibrated at our factory and the volume SD is 0.6%.
SDhandling measures the deviation from sample handling. This varies from lab to lab depending on the skill of
the operator, but poor sample handling leads to large variations in both manual and automatic cell
counting.
Other contributors (SDother) also add to the total variation, and it is therefore important to know these
contributors when wanting to decrease sources of error and optimize cell counting reliability. One
contributor could be the precision of the pipetting volume, which is dependent on how well the pipettes
have been calibrated.
Manual cell counting
Manual cell counting using a hemocytometer and trypan blue staining has been the universal standard for
determining cell concentration and viability for decades. Using this method gives considerably higher SDs
and CVs of cell counts compared to those when using the NucleoCounter® NC-202™
1
. Typically, fewer cells
are counted in manual counting
2
and therefore the contribution from the Poisson distribution to the total
variation will be greater. As such, the reproducibility of the hemocytometer is highly user-dependent,
whereas the Via2-Cassette™ provides an option of sample processing and staining independent of the
operator. The hemocytometer requires the operator to be familiar with its correct use in order to avoid
often large variations.
1
See 994-2029 Tech Note NucleoCounter® NC-202™ Performance for full data (available online at
www.chemometec.com/NC-202)
2
Generally, it is recommended that you count at least 100 cells for hemocytometers, but users tend to count only
100-200 cells. This results in a Poisson contribution to the CV of 7-10% in addition to the other sources of variation.
E.g. if 150 cells are counted in 5 big squares of a hemocytometer using trypan blue 1:1, the cell concentration will be
(150 cells / 0.5 µl) ×2 = 6 ×105cells/ml with an SD of 4.9 ×104cells/ml (CV 8.1%). Analyzing the same sample on the
NucleoCounter® NC-202™ will result in counting 812 cells with an SD of 2.1 ×104cells/ml (CV 3.5%). These calculations
only take the contribution from Poisson distribution into consideration.
Page 12 of 127

Doc. No.: 994-2030 v. 1.0 · Issue date: 04-Apr-2020
Page 3 of 3
ChemoMetec A/S · Gydevang 43 · 3450 Allerod · Denmark · suppor[email protected] · www.chemometec.com
User-dependent deviation also occurs in manual counting when recording an event, i.e. defining a cell to be
counted, making manual counting highly subjective. One operator may include an object that another
operator may discard during cell counting. For instance, different operators may have different perceptions
of what exactly defines a cell, the specific borders to count within, or whether a cell stains positive for
trypan blue and is thereby defined as dead. The NucleoCounter® NC-202™ is completely objective without
these sources of variation, therefore providing much more reliable results.
Page 13 of 127

Page 14 of 127

Page 15 of 127

Page 16 of 127

Doc. No. 992-3101 v. 1.1 ∙ Issue date: 26-Apr-2019 Page 1 of 1
Template 880-0011-79C v. 1
ChemoMetec A/S ∙ Gydevang 43 ∙ DK-3450 Allerød ∙ Denmark ∙ support@chemometec.com ∙ www.chemometec.com
941-0024 Via2-CassetteTM 10 x 10 Unit
Contents
Box with 100 Via2-Cassettes™ (10 bags each containing 10 Via2-Cassettes™).
Application
The Via2-Cassette™ is used together with the NucleoCounter® Instruments to determine total cell count and
non-viable cell count in cell cultures.
If the Via2-Cassettes™ are used with the NucleoCounter® NC-200™, a specific Via2 protocol is
required, and NucleoView™ should be updated to version 1.3.0.0 or higher.
Usage
The Via2-Cassette™ is a plastic device that combines cell sample aspiration and cell staining in a single unit.
The Via2-Cassette™ features a piston, a flow system with
deposited Acridine Orange and DAPI, and a
measurementchamber.
The closed cassette system ensures that the operator will not be exposed to the
dyes and the device can be safely disposed.
The cassette is loaded by gently pressing the piston while the tip of the cassette is immersed into the cell
sample. Approximately 60 µl of sample mixture is loaded into the cassette.
AO and DAPI are deposited as salt
crystals in the first three lanes of the flow system. The dyes are dissolved by the cell sample buffer mixture
and will stain all cells and non-viable cells, respectively.
The loaded cassette is placed in the instrument immediately and the analysis is initiated using the
appropriate software. During analysis the NucleoCounter® instrument pushes the piston further into the
cylinder of the cassette to mix the samples, load the measurement chamber and acquire the fluorescence
image. Each cassette carries a dot-code, that indicates the exact volume of its counting chamber. This
dot-code is read by the NucleoCounter® instrument and is used by the software to calculate the cell
concentration.
Caution!
In order to avoid contaminating the clear window of the measurement chamber it is important
not to touch the window when handling the cassettes.
For a detailed procedure of the analysis refer to the User's Guide for the instrument and the appropriate
Application Notes.
Stability
Forsealedbags,the expirydateisshown onthe bagandonthe box.TheVia2-Cassettes™areproduced15
months before the expiration date.
The cassettes expire 2 weeks after opening the bag. Protect the cassettes against moisture and light.
Storage
Store cassettes at room temperature (< 40°C).
Safety Information
Each Via2-Cassette™ contains 0.2 µg of Acridine Orange (AO) and 0.6 µg of 4',6-diamidi-
no-2-
phenylindole(DAPI)deposited ineachcassette duringmanufacture. Whenthecassetteis
loaded with a
sample mixture the reagents are dissolved in the mixture. The concentration of AO and DAPI in the
mixture will be approximately 3.3 and 9.9 mg/l, respectively.
Never attempt to unload the sample from the cassette. This action can result in release of potentially
harmful substances and biohazardous material from the sample/reagent mixture.
Avoid physical contact with the cassette tip, which may be contaminated by harmful substances and
biohazardous material from the sample mixture.
Use gloves and suitable protective clothing as per local requirements.
Disposal of Waste
After use, the Via2-Cassette™ should be disposed of in accordance with applicable National, Federal, Local
and State laws and regulations.
Legislation addressing waste disposal requirements may differ by country, state and/ or territory. Each
user must refer to laws operating in their area. In some areas, certain wastes must be tracked.
Page 17 of 127

--------------------------------------------------------------------------------------------------------------------------------------------------------------------------
* 941-0002 NucleoCassette –10 x 10 units
941-0006 SP1-Cassette –10 x 10 units
941-0008 SCC-Cassette –10 x 10 units
941-0016 PI-Cassette –10 x 10 units
941-0012 Via1-Cassette –10 x 10 units
941-0024 Via2-Cassette –10 x 10 units
995-0053 PI Reagent, SDS
995-0051 Via1-Reagent, SDS
995-0065 Via2-Reagent, SDS
Chemometec
Gydevang 43
DK-3450 Allerød
Denmark
Att.: To whom it may concern
Ref:
11822339
Init:
TWS
Date:
9 March 2018
Conformity Statement on the Chemometec NucleoCassette™, SP1-Cassette™, SCC-
Cassette™, PI-Cassette™, Via1-Cassette™and Via2-Cassette™
This is a statement on the conformity of the NucleoCassette™, SP1-Cassette™, SCC-Cassette™, PI-
Cassette™Via1-Cassette™and Via2-Cassette™(hereafter referred to as: the Cassette)
manufactured by Chemometec with current legislation regarding labelling obligations.
The Cassette is a custom-made disposable plastic device designed for optimal sample handling and
safe disposal. It is preloaded with chemical reactants used for staining cells loaded from a sample
mixture. The sample and the chemical reactants are fully contained within the Cassette and will not be
released during use, and the Cassette including the sample can safely be disposed of after use.
Based on Guidance on requirements for substances in articles (Version 4.0, June 2017) published by
the European Chemicals Agency (ECHA) and product descriptions provided by Chemometec* it is
assessed that the Cassette with its content of chemical reactants is regarded as an article under
Regulation 1907/2006 (REACH-regulation). Consequently, the Cassettes and their secondary
packaging need not to be classified and labelled according to the CLP-regulation (Regulation No
1272/2008) and according to the REACH-regulation, a safety datasheet (SDS) for the chemical
reactants is not required.
It should be noted that according to the above-mentioned REACH-regulation, manufacturers and
importers must notify ECHA if an article contains a “substance of very high concern” (SVHC) at a
concentration of 0.1% weight by weight and if the total manufacture/import quantity of this substance
exceeds one tonne per year.
Sincerely,
09-03-2018
X
Torben Wilde Schou, MSc, PhD
Scientific Advisor
Signed by: Torben Wilde Schou
DHI
Agern Allé 5
DK-2970 Hørsholm
Denmark
+45 4516 9200 Telephone
+45 4516 9292 Telefax
dhi@dhigroup.com
www.dhigroup.com
Page 18 of 127

1
2
3
Page 1 of 1
Doc. No.: 992-3102 v. 2.0
ChemoMetec A/S ∙ Gydevang 43 ∙ DK-3450 Allerod ∙ Denmark ∙ Support@chemometec.com ∙ www.chemometec.com
941-0024 Via2-Cassette™
Certificate of Analysis
This lot was released by:
E.g. Thea Birkefeldt, QC Manager
This fixed max. value is independent of the results from the testing of reference cassettes.
pp=percentage points
All cassette lots are composed of one or more sub-lots. Each production day represents a sub-lot. All sub-lots are tested
together but evaluated separately. The sub-lots are denoted A to G, and this sub-lot letter is printed on the box-label.
Result of QC
test
Sub-Lot #DIV/0! #DIV/0! #DIV/0! PASSED
Viability
Accept Criteria Ref. Cass. +/- 5.0 % Fixed Max. Value1: 6.0 % Ref. Cass. +/- 4.0 pp2
Accept Range
#DIV/0!
0.0-6.0 %
#DIV/0!
Sub-Lot 3data:
Lot mmyy-xx
Total Cell Count
(x10
6
Cells/ml)
Coefficient of Variation
Total Cell Count
(x10
6
Cells/ml)
Coefficient of Variation Viability
This lot has been tested according to the internal ChemoMetec® quality procedures. The Quality Test is composed of a
physical qualification followed by a quantitative test of the cassettes. The results of the quantitative test are presented below.
This lot meets or exceeds all product specifications.
Reference Cassette data:
Total Cell Count
(x10
6
Cells/ml)
Coefficient of Variation Viability
Ref. Cass. #DIV/0! #DIV/0! #DIV/0!
Accept Ranges based on Reference Cassette data:
The Via2-Cassette™ is part of the NucleoCounter® system. The cassette is used to determine total cell
count and non-viable cell count in mammalian cell cultures. Only a single cassette is used for estimating
both the total count and the non-viable count.
Lot number: mmyy-xx
Release date:
Mmm-yyyy
Exp. Date: Mmm-yyyy
Application:
EXAMPLE
Page 19 of 127

880-0030-76A
Quality Assurance Measures in the
Production of the ChemoMetec Cassettes
V 4.0
Contents
Document Approval............................................................................................................................................2
Related Documentation .....................................................................................................................................2
Revision History..................................................................................................................................................2
Product and Service Inputs:............................................................................................................................3
Key Characteristics/Specifications:.................................................................................................................3
Inspection Points and Monitoring Metrics:....................................................................................................3
Entrance Control of Raw Material:.............................................................................................................3
Production:.................................................................................................................................................3
Quality Control Test of Finished Cassettes:....................................................................................................5
Training Documents: ......................................................................................................................................5
Product and Service Outputs:.........................................................................................................................5
Page 20 of 127
Other manuals for NucleoCounter NC-202
2
Table of contents
Other ChemoMetec Cash Counter manuals