ChemoMetec NucleoCounter User manual

NucleoCounter®
User's Guide
P/N 991-0009
Revision 1.4
Technology that counts

i
This page was intentionally left blank

ii
NucleoCounter
Mammalian Cell Counter
P/N 991-0009 (English)
Revision 1.3
June, 2015
ChemoMetec A/S
Gydevang 43, DK-3450 Allerød, Denmark
Telephone: (+45) 48 13 10 20, Fax: (+45) 48 13 10
21
Internet: www.chemometec.com,
E-mail:info@chemometec.com

iii
This page was intentionally left blank

iv
Caution!
This equipment must be operated as described in this User’ s Guide. Please
read the entire guide before attempting to use this unit. Please pay attention
to that gloves or protective clothing are not worn on the
illustrations/pictures shown in this User’s Guide. However, ChemoMetec A/S
does recommend that the user wear suitable protective clothing etc.
Contacting support
Technical information including product literature, answers to questions
regarding the operation of the NucleoCounternot covered in this document and
information on software upgrade is available through the following:
For e-mail support, send questions to NucleoCounterTechnical Support
on the address support@chemometec.com
To speak with a Technical Support Specialist, call (+45) 48 13 10 20.
Please note the NucleoCounterserial number and have it available when
contacting ChemoMetec A/S for support. The NucleoCounterserial number is
found on the label affixed to the bottom of the instrument and in the display
upon start-up.
Sales and ordering information
For sales assistance with NucleoCounteror the NucleoViewsoftware, to place
an order for a NucleoCounteror consumables, call (+45) 48 13 10 20, fax
(+45) 48 13 10 21, or send e-mail to sales@chemometec.com
Disclaimer Notices
The material in this document is for information only and is subject to change
without notice. While reasonable efforts have been made in the preparation of
this document to assure its accuracy, ChemoMetec A/S assumes no liability
resulting from errors or omissions in this document, or from the use of the
information contained herein.
ChemoMetec A/S reserves the right to make changes in the product design
without reservation and without notification to its users.
Copyright Notices
Copyright © ChemoMetec A/S 2005. All rights reserved. No part of this
publication may be reproduced, stored in a retrieval system, or transmitted in
any form or by any means, electronic, mechanical, photocopying, recording or
otherwise, without the prior written consent of ChemoMetec A/S, Gydevang 43,
DK-3450 Allerød, Denmark.
ChemoMetec and NucleoCounter are registered trademarks owned by ChemoMetec
A/S. NucleoCounter , NucleoCassettes, Reagent A100, Reagent B and NucleoView
are trademarks of ChemoMetec A/S.
All other trademarks are the property of their respective owners.

v
This page was intentionally left blank

vi
Declaration of Conformity
Name of product: NucleoCounter
Type: Mammalian Cells
Other identifying data: Part no. 900-0004
The product complies with requirements of the following
directives:
89/336/EEC - Electromagnetic Compatibility (EMC)
89/392/EEC - Machinery Directive
Harmonized standards, which have been used:
EN61326: 1997+A1: 1998 (Class B) +A2: 2000, Electrical
equipment for measurement, control and laboratory use –EMC
requirements (emission and immunity). Annex B and C from A1:
1998 is used.
This device complies with Part 15 of the FCC Rules. Operation
is subject to the following two conditions: (1) this device may
not cause harmful interference, and (2) this device must accept
any interference received, including interference that may
cause undesired operation.
Date: 2013-11-
07
Signed:
Name: Sarah R. Rasmussen
Position: QA/QC Managers
Name and address of manufacturer:
ChemoMetec A/S
Gydevang 43
DK-3450 Allerod
DENMARK

vii
This page was intentionally left blank
viii
WEEE directive information –Europe only
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment) - Europe only
This marking shown on the product or its literature,
indicates that it should not be disposed together
with other household wastes at the end of its working
life. To prevent possible harm to the environment or
human health from uncontrolled waste disposal, please
separate this from other types of wastes and recycle
it responsibly to promote the sustainable reuse of
material resources.
Business users should contact their supplier and
check the terms and conditions of the purchase
contract. This product should not be mixed with other
commercial wastes for disposal.
This information is listed in “Appendix 3: WEEE
directive information in more EU languages”.

ix
This page was intentionally left blank
Introduction and intended use
x
Introduction and intended use
The NucleoCounter®is a novel instrument designed specially to
count mammalian cells. The instrument is a part of the
NucleoCounter system, which also comprises the NucleoCassette™,
Lysis buffer (Reagent A100) and Stabilizing buffer (Reagent B).
The NucleoCounter system allows reliable, fast and objective
cell counting to be carried out based on the method of
fluorescence microscopy. It also makes it possible for the user
to stain and count mammalian cells without handling hazardous
dyes.
The NucleoCounter system has been developed to count mammalian
cells in suspension. The mammalian cells could be cultured in
T-flasks, bioreactors or on the surface of micro carriers.
The NucleoCounter is developed as a stand-alone instrument but
optionally it can be connected to a computer via an USB-
interface. When connected to a computer the NucleoViewTM
software offers various features such as documentation of the
obtained results. Read more about the software in the
NucleoViewTM User´s Guide.
The NucleoCounter type at ChemoMetec A/S is 900-0004.
This user´s guide is valid for NucleoCounter software
version 4.XX
The NucleoCounter system enables the user to obtain cell counts
and viability determinations on cell suspensions from a wide
range of cultured mammalian cell lines.
The cell counts and viability determination rest on the ability
of the fluorescent dye to differentiate between viable and non-
viable cells by means of different permeability of the plasma
membranes. Thereby the concentration of viable and non-viable
cells in suspension may be determined using the NucleoCounter
system.

Introduction and intended use
xi
The NucleoCounter system is intended for fast, reliable and
efficient cell counting and viability determinations of samples
containing mammalian cells.
The NucleoCounter, NucleoCassette™, the Lysis buffer and the
Stabilizing buffer are not intended for human or veterinary
consumption.
The NucleoCounter system is for research use only, not for
diagnostic use.

Warnings and precautions
xiii
Warnings and precautions
Wherever the symbol appears on the NucleoCounter instrument,
it indicates that the manual must be consulted for precautions
and warnings.
Power and cables
Use the shielded USB cable supplied with the NucleoCounter to
ensure that you maintain the appropriate EMI classification for
the intended environment.
The USB interface connector of the NucleoCounter must only
be connected to SELV circuits. External computing devices
connected to the USB interface connector of the NucleoCounter
has to comply with the standards, UL 1950 and IEC/EN 60950.
Table 1.Description of the NucleoCounter USB interface
connector
Use the shielded printer cable supplied with the NucleoCounter
to ensure that you maintain the appropriate EMI classification
for the intended environment.
The printer interface connector of the NucleoCounter must
only be connected to the printer supplied by ChemoMetec A/S.
External computing devices connected to the printer interface
connector of the NucleoCounter has to comply with the
standards, UL 1950 and IEC/EN 60950.
1
In normal operation mode (refers to Pin no 4)
Pin no.
Name
Maximum
Voltage level1
1
+5V
+5
VDC
2
D-
+3.5
VDC
3
D+
+3.5
VDC
4
DGND
0
VDC
Metal
enclosure
Shield
(connected
to DGND)
0
VDC
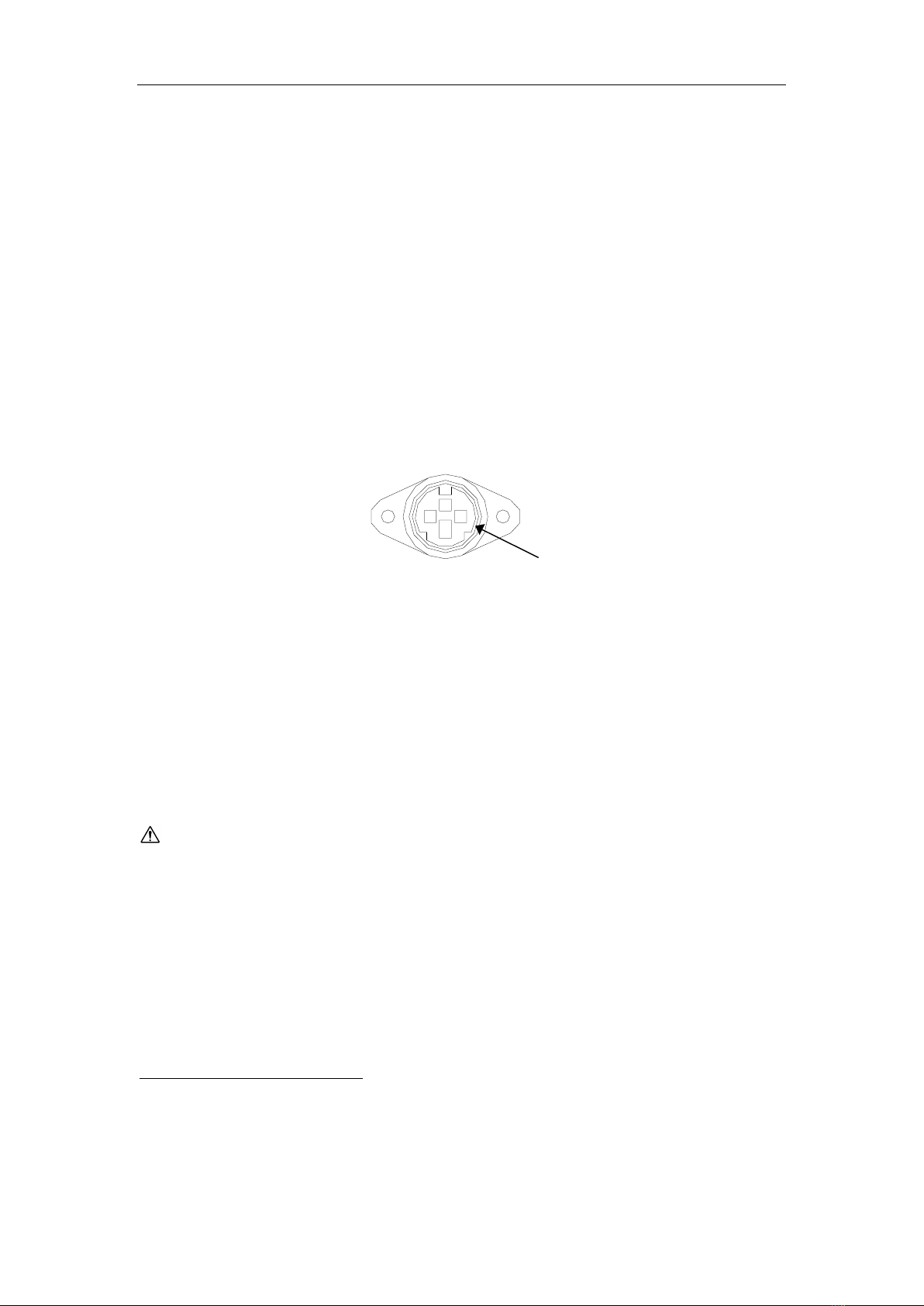
Warnings and precautions
xiv
Table 2 Description of the NucleoCounter Printer Output
connector
Pin no
Name
Maximum Voltage
level2
13
2
Rx
±10 VDC
3
Tx
±10 VDC
Metal
Enclosure
DGND
0 VDC
2
1
3
Metal
Enclosure
Figure 1 Printer Output connector seen from the cable entry
side.
NucleoCounter is powered by an external 12VDC power supply. For
safe use, please follow the instructions for connecting the
power supply.
The NucleoCounter shall only be used with one of the
following external power supplies:
Power supply Class I, Proton Electronic Industrial Co.
Ltd., model SPN-260-12, input rated 100-240 Vac, 50-60Hz,
1.6 A. Output rated 12 Vdc, 4.3 A.
Power Supply Class I, Powerbox Europe AB, model EBH 03
131, input rated 100-240 Vac, 47-63 Hz, 0.8A. Output
rated 11-13 Vdc, 2.7 A.
2
In normal operation. Refers to DGND Metal Enclosure. Taken
from the datasheet for MAX202ECSE.
3
There is no signal connected to pin 1.

Warnings and precautions
xv
Power supply Class II, Celetron USA Inc., model
ZVC40LT12E, input rated 100-240 Vac, 50-60Hz, 1.2 A.
Output rated 12 Vdc, 3.3 A.
The detachable power supply cord set and appliance inlet of
the external power supply are considered as the disconnecting
device.
The mains supply cord and plug of the external power supply
shall comply with any national regulations.
The user shall be made aware of that, if the NucleoCounter
and the external power supply is used in a manner not specified
by the manufacturer, the protection provided by the
NucleoCounter and the external power supply may be impaired.
Electromagnetic interference
NOTE: This equipment has been tested and found to comply with
the limits for a Class B digital device, pursuant to Part 15 of
the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate
radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful
interference to radio communications. However, there is no
guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference
to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try
to correct the interference by one or more of the following
measures:
Reorient or relocate the receiving antenna.
Increase the separation between the equipment and
receiver.
Connect the equipment to an outlet on a circuit different
from that to which the receiver is connected.
Consult the dealer or an experienced radio/TV technician
for help.
Caution! Changes or modifications not expressly approved by the
party responsible for compliance could void the user's
authority to operate the equipment.

Warnings and precautions
xvi
Cassettes, Reagents and Dispensers
With respect to usage and handling of cassettes, lysis buffers
and dispensers, please refer to appropriate package inserts for
these items.
Caution! When using a bottle-top dispenser: To protect against
accidental splashes protective clothing, eye protection and
gloves must be worn when using potentially hazardous liquids.
General
Any biological specimen should be handled as if it is capable
of transmitting infectious disease and disposed of with proper
precautions according with federal, state and local
regulations.
Avoid specimen contact with skin or mucous membranes.
Never pipette by mouth.
Avoid cross contamination of the samples when preparing of the
samples. This can compromise the quality of the results.

1 Inspection and Unpacking of Equipment
1
Table of contents
DECLARATION OF CONFORMITY .......................................................................VI
WEEE DIRECTIVE INFORMATION –EUROPE ONLY .........................................VIII
INTRODUCTION AND INTENDED USE ................................................................. X
WARNINGS AND PRECAUTIONS.......................................................................XIII
1INSPECTION AND UNPACKING OF EQUIPMENT ........................................... 3
2INTRODUCING THE NUCLEOCOUNTER ........................................................ 5
2.1 THE NUCLEOCOUNTER INSTRUMENT .............................................................. 5
2.1.1 Fluorescence microscope ............................. 6
2.1.2 Built-in image analysis ............................. 7
2.2 THE NUCLEOCASSETTE ............................................................................... 8
2.2.1 Loading the cassette ................................ 8
2.2.2 Fluorescent dye ..................................... 8
2.2.3 Sample volume ....................................... 9
2.2.4 Cassette handling ................................... 9
2.3 LYSIS BUFFER (REAGENT A100) AND STABILIZING BUFFER (REAGENT B) ........... 9
3CONTROL BUTTONS, KEYPAD AND DISPLAY ..............................................12
3.1 INTERACTIVE CONTROLS .............................................................................12
3.1.1 Control Buttons .................................... 12
3.1.2 Keypad ............................................. 13
3.2 INTERFACE DISPLAY ...................................................................................13
3.2.1 Status indicator ................................... 13
3.2.2 Display screen ..................................... 13
4INSTALLATION AND START-UP....................................................................14
4.1 POWER ON,POWER OFF ..............................................................................14
4.1.1 Starting up ........................................ 14
4.1.2 Shutting down ...................................... 15
4.2 INSTALLATION OF NUCLEOVIEW ...................................................................15
5OPERATION OF THE NUCLEOCOUNTER......................................................16
5.1 READY MODE .............................................................................................16
5.2 INSERTING AND REMOVING THE CASSETTE .....................................................16
5.2.1 Protection of the optical system ................... 16
5.2.2 Inserting cassette ................................. 16
5.3 MEASURING -THE RUN BUTTON ...................................................................17
5.3.1 Actuator movements ................................. 17
5.3.2 Result presentation ................................ 18
5.3.3 External computer .................................. 18
5.4 RESULT DISPLAY ........................................................................................18
5.4.1 Result mode ........................................ 18
5.4.2 Saving parameters .................................. 19

1 Inspection and Unpacking of Equipment
2
5.4.3 Setting Date (F200) and Time (F201) ................ 20
5.4.4 Reset Counter (F30) ................................ 20
5.4.5 LCD contrast (F220) ................................ 21
5.4.6 Instrument Info (F100) ............................. 21
5.5 ZERO COUNT CHECK (F50) .........................................................................22
6TOTAL AND VIABILITY COUNT .....................................................................26
6.1 TOTAL CELL COUNT ....................................................................................27
6.2 COUNTING NON-VIABLE CELLS......................................................................29
6.3 CALCULATE VIABILITY .................................................................................29
6.4 DILUTE THE SAMPLES .................................................................................30
7MAINTENANCE OF NUCLEOCOUNTER.........................................................33
7.1 CLEANING .................................................................................................33
7.1.1 Cassette insertion area ............................ 33
7.1.2 Optical elements ................................... 33
7.1.3 Removal of dust particles .......................... 34
7.1.4 Spill of liquid .................................... 35
8TROUBLESHOOTING - ERROR MESSAGES..................................................37
8.1 NO VALID CASSETTE ...................................................................................37
8.2 ANALYSIS ABORTED ....................................................................................37
8.3 ACTUATOR ERROR MESSAGES......................................................................38
8.4 SAMPLE COULD NOT BE ANALYZED................................................................38
8.5 SENSOR ERROR .........................................................................................39
8.6 POWER-ON FAILURE ...................................................................................39
9TECHNICAL SPECIFICATIONS......................................................................42
9.1 THE NUCLEOCOUNTER ...............................................................................42
9.2 THE NUCLEOCASSETTE™...........................................................................43
9.3 EMC/EMI STANDARDS................................................................................43
10 EQUIPMENT AND ACCESSORIES..............................................................44
10.1 EQUIPMENT AND ACCESSORIES LIST..........................................................44
10.2 POWER SUPPLY ......................................................................................45
10.3 MAINS POWER CORD ...............................................................................46
10.4 DRY COMPRESSED AIR .............................................................................47
10.5 EXTERNAL PRINTER (OPTIONAL)................................................................47
10.6 NUCLEOCOUNTER TEST KIT .....................................................................49
10.7 NUCLEOVIEW .........................................................................................49
11 APPENDIX 1: RESULT MODE 4..................................................................51
12 APPENDIX 2: EXTENDED NUCLEOCOUNTER®NC-100TM SETTINGS ........53
13 APPENDIX 3: WEEE DIRECTIVE INFORMATION IN MORE EU LANGUAGES
56

1 Inspection and Unpacking of Equipment
3
1 Inspection and Unpacking of Equipment
Upon receiving the order from ChemoMetec A/S, the box or boxes
should be carefully inspected for any damage that may have
occurred during shipping. Any damage must be reported to the
carrier and to ChemoMetec A/S immediately.
Unpack the order, saving the packing materials for possible
later use. Also be sure to save the User’s Guide, for
instruction and reference.
Verify the ChemoMetec packing list received refers to the
correct and ordered materials, and that nothing is missing.
If any part of the order was damaged during shipping or is
missing, or fails to operate, please contact ChemoMetec A/S.
Table of contents
Other ChemoMetec Cash Counter manuals