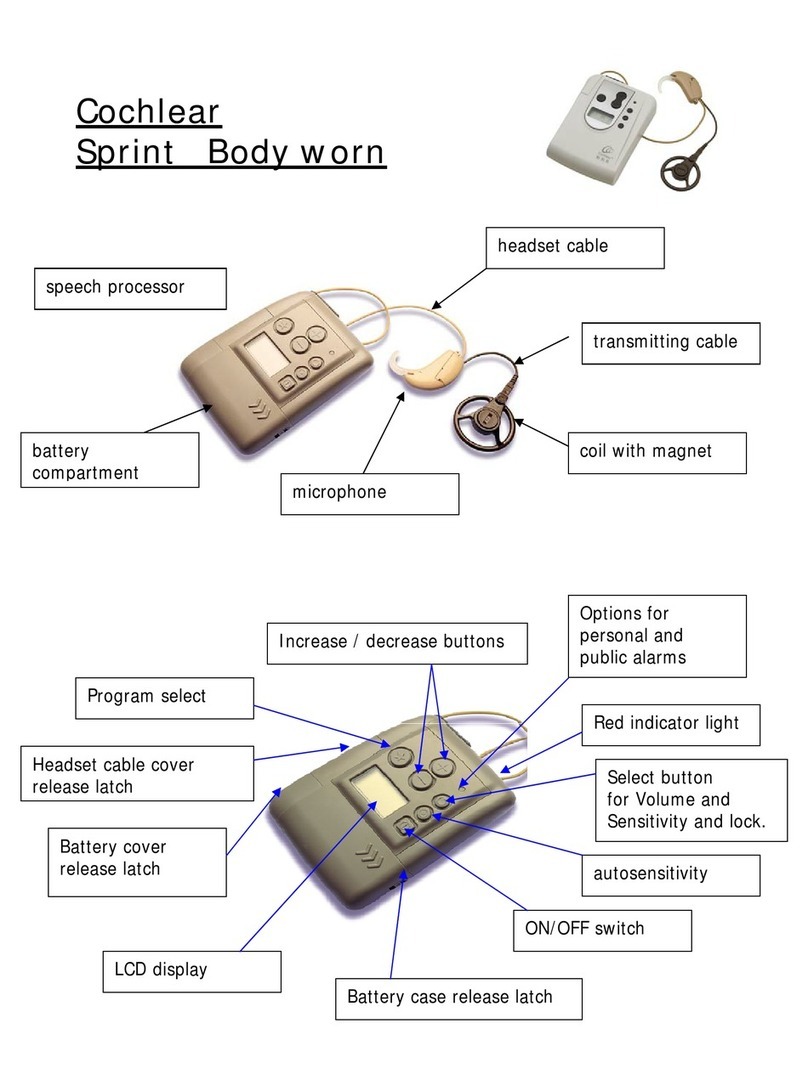
Sound processor type designations for
models included in this User Manual are:
FCC ID: QZ3BAHA5SUP, IC: 8039C-BAHA5SUP, IC model:
Baha®5 SUP.
Statement:
This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions:
(1) this device may not cause harmful interference, and
(2) this device must accept any interference received,
including interference that may cause undesired operation.
Note: This equipment has been tested and found to
comply with the limits for a Class B digital device, pursuant
to part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference
in a residential installation. This equipment generates, uses
and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause
harmful interference to radio communications. However,
there is no guarantee that interference will not occur in
a particular installation. If this equipment does cause
harmful interference to radio or television reception, which
can be determined by turning the equipment off and on,
the user is encouraged to try to correct the interference by
one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and
receiver.
• Connect the equipment into an outlet on a circuit
different from the one in which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician
for help.
• Changes or modifications can void the user’s authority to
operate the equipment.
Intended use
The Cochlear™Baha®5 SuperPower uses bone conduction
to transmit sounds to the cochlea (inner ear). It is indicated
for people with conductive hearing loss, mixed hearing
loss and single sided sensorineural deafness (SSD).
Furthermore it is indicated for bilateral and paediatric
recipients. Fitting range up to 65 dB SNHL. It works by
combining a sound processor and a small titanium implant
that is placed in the skull behind the ear. The skull bone
integrates with the titanium implant through a process
called osseointegration. This allows sound to be conducted
via the skull bone directly to the cochlea, which improves
hearing performance. The sound processor can be used
together with the Baha Softband.
The fitting is to be done either at a hospital, by an
audiologist, or in some countries, by a hearing care
professional.
List of countries:
Not all products are available in all markets. Product
availability is subject to regulatory approval in the
respective markets.
The products are in compliance with the following
regulatory requirements:
• In EU: the device conforms to the Essential
Requirements according to Annex I of Council Directive
93/42/EEC for medical devices (MDD) and essential
requirements and other relevant provisions of Directive
2014/53/EU (RED). The declaration of conformity may
be consulted at www.cochlear.com.
• Other identified applicable international regulatory
requirements in countries outside the EU and US. Please
refer to local country requirements for these areas.
• In Canada the sound processor is certified under the
following certification number: IC: 8039C-BAHA5SUP
and model no.: IC model: Baha®5 SUP.
• This device complies with Industry Canada licence-
exempt RSS standard(s).
• This Class B digital apparatus complies with Canadian
ICES-003. Cet appareil numérique de la classe B est
conforme à la norme NMB-003 du Canada.
• Operation is subject to the following two conditions:
(1) this device may not cause interference, and (2)
this device must accept any interference, including
interference that may cause undesired operation
of the device. L’exploitation est autorisée aux deux
conditions suivantes : (1) l’appareil ne doit pas produire
de brouillage, et (2) l’utilisateur de l’appareil doit
accepter tout brouillage radioélectrique subi, même
si le brouillage est susceptible d’en compromettre le
fonctionnement.
Equipment includes RF transmitter
Note: The sound processor is suited for use
in a home healthcare environment. The
home healthcare environment includes locations such as
homes, schools, churches, restaurants, hotels, cars, and
airplanes, where equipment and systems are less likely to
be administered by healthcare professionals.
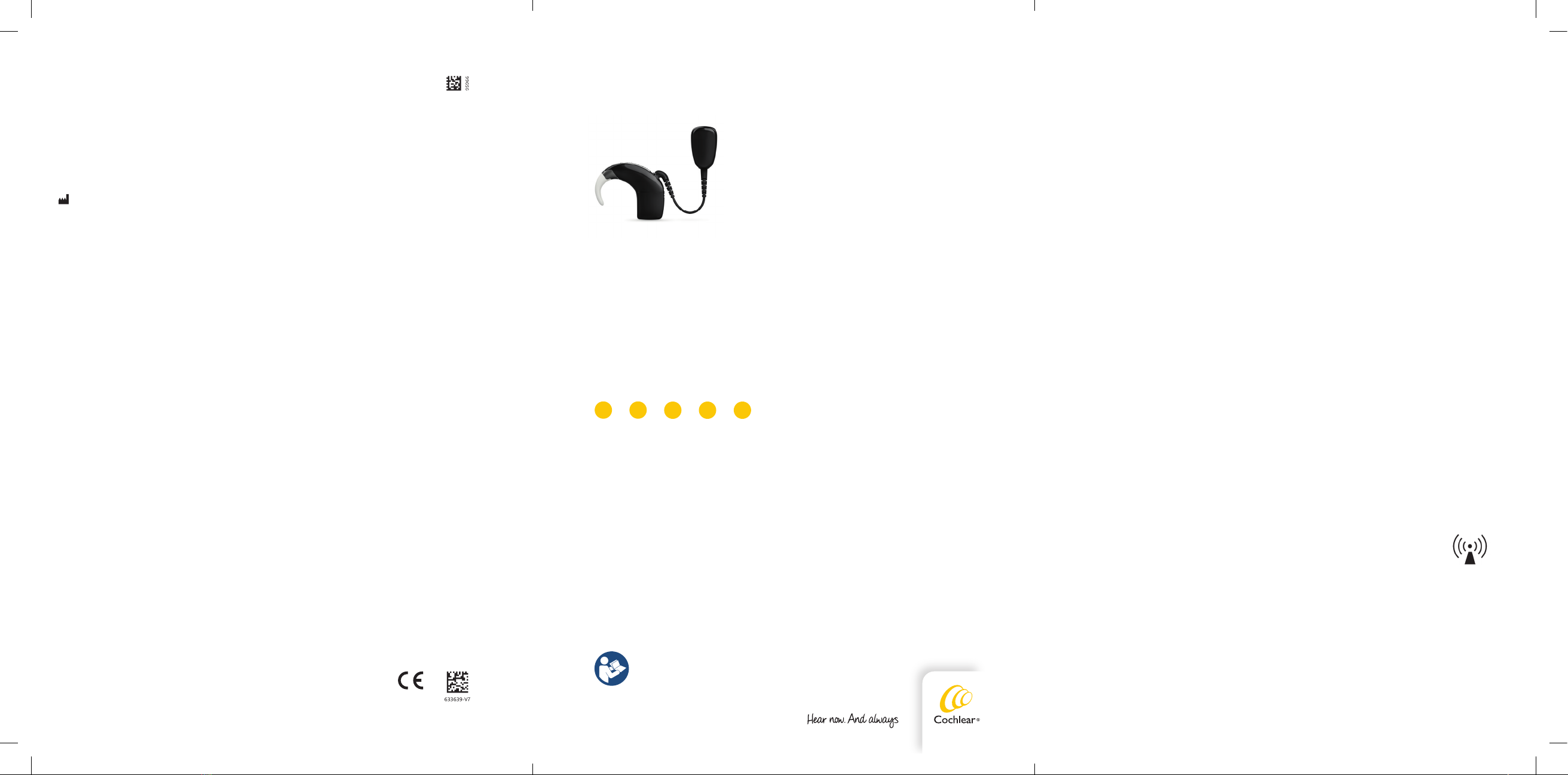
Cochlear™
Baha® 5 SuperPower Sound Processor
ZONE 2
English
Español
Ελληνικά
Português
תירבע
315 27 39 51
EN-GB ES EL PT HE
User manual part A
Cochlear Bone Anchored Solutions AB, Konstruktionsvägen 14, 435 33 Mölnlycke, Sweden
Tel: +46 31 792 44 00, Fax: +46 31 792 46 95
Regional Offices
Cochlear Ltd, (ABN 96 002 618 073), 1 University Avenue,
Macquarie University, NSW 2109 Australia
Tel: +61 2 9428 6555, Fax: +61 2 9428 6352
Cochlear Americas, 10350 Park Meadows Drive, Lone Tree,
CO 80124, USA
Tel: +1 303 790 9010, Fax: +1 303 792 9025
Cochlear AG, EMEA Headquarters, Peter Merian-Weg 4, 4052 Basel,
Switzerland
Tel: +41 61 205 8204, Fax: +41 61 205 8205
Cochlear Latinoamerica, S. A., International Business Park
Building 3835, Office 403 Panama Pacifico, Panama
Tel: +507 830 6220, Fax: +507 830 6218
Local Offices
Cochlear Europe Ltd, 6 Dashwood Lang Road, Bourne Business Park,
Addlestone, Surrey, KT15 2HJ, United Kingdom
Tel: +44 1932 26 3400, Fax: +44 1932 26 3426
Cochlear Deutschland GmbH & Co. KG, Karl-Wiechert-Allee 76A,
30625 Hannover, Germany
Tel: +49 511 542 770, Fax: +49 511 542 7770
Cochlear Benelux NV, Schaliënhoevedreef 20 I, 2800 Mechelen,
Belgium
Tel: +32 15 79 55 77, Fax: +32 15 79 55 70
Cochlear France S.A.S., 135 route de Saint Simon, 31035 Toulouse,
France
Tel: +33 5 34 63 85 85 (international), Tel: 0805 200 016 (national),
Fax: +33 5 34 63 85 80
Cochlear Italia SRL, Via Larga 33, 40138 Bologna, Italy
Tel: +39 051 601 53 11, Fax: +39 051 392 062
Cochlear Tıbbi Cihazlar ve Sağlık Hizmetleri Ltd. Sti.,
Cubuklu Mah. Bogazici Cad. Bogazici Plaza, No: 6/1 Kavacik,
TR-34805 Beykoz-Istanbul, Turkey
Tel: +90 216 538 59 00, Fax: +90 216 538 59 19
Cochlear Nordic AB, Konstruktionsvägen 14, 435 33 Mölnlycke,
Sweden
Tel: +46 31 335 14 61, Fax: +46 31 335 14 60
Cochlear Canada Inc, 2500-120 Adelaide Street West, Toronto,
ON M5H 1T1, Canada
Tel: +1 416 972 5082, Fax: +1 416 972 5083
Nihon Cochlear Co Ltd, Ochanomizu-Motomachi Bldg 2-3-7 Hongo,
Bunkyo-Ku, Tokyo 113-0033, Japan
Tel: +81 3 3817 0241, Fax: +81 3 3817 0245
Cochlear Limited (Singapore Branch), 238A Thomson Road #25-06,
Novena Square Office Tower A, Singapore 307684, Singapore
Phone: +65 65533814, Fax: +65 64514105
Cochlear Medical Device (Beijing) Co Ltd, Unit 2608-2617,
26th Floor,No.9 Building, No.91 Jianguo Road, Chaoyang District,
Beijing 100022, PR China
Tel: +86 10 5909 7800, Fax: +86 10 5909 7900
Cochlear (HK) Ltd, Room 1404-1406, 14/F, Leighton Centre,
77 Leighton Road, Causeway Bay, Hong Kong
Tel: +852 2530 5773, Fax: +852 2530 5183
Cochlear Korea Ltd, 1st floor, Cheongwon Building 33,
Teheran-ro 8 gil, Gangnam-gu, Seoul, Korea
Tel: +82 2 533 4450, Fax: +82 2 533 8408
Cochlear Medical Device Company India PVT Ltd,
Platina Bldg, Ground Floor, Plot No. C 59, G Block, BKC, Bandra East,
Mumbai 400051 India
Tel: +91 22 6112 1111, Fax: +91 22 61121100
Cochlear Colombia, Avenida Carrera 9 #115-06
Of. 1201 Edificio Tierra Firme, Bogota D.C., Colombia
Tel: +57 315 339 7169 / +57 315 332 5483
Cochlear México, SS.A. de C.V, Av. Tamaulipas 150 Torre A piso 9,
Col. Hipódromo Condesa, 06170 Cuauhtémoc, Ciudad de México,
México
Tel: +52 0155 5256 2199
www.cochlear.com
Please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional
will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available
in all countries. Please contact your local Cochlear representative for product information. Cochlear Baha 5 sound processors are
compatible with iPhone, iPad and iPod touch. For compatibility information visit www.cochlear.com/compatibility.
Cochlear, Baha, 科利耳, コクレア , 코클리어, Hear now. And always, SmartSound, the elliptical logo, and marks bearing an ® or ™ symbol,
are either trademarks or registered trademarks of Cochlear Bone Anchored Solutions AB or Cochlear Limited (unless otherwise noted).
The Bluetooth word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Cochlear
Limited is under license. Apple, the Apple logo, FaceTime, iPhone, iPad and iPod touch are trademarks of Apple Inc., registered in the U.S.
and other countries. App Store is a service mark of Apple Inc.
© Cochlear Bone Anchored Solutions AB 2020. All rights reserved. 2020-09.
95966 633639-V7. Translation of 631286-V11.