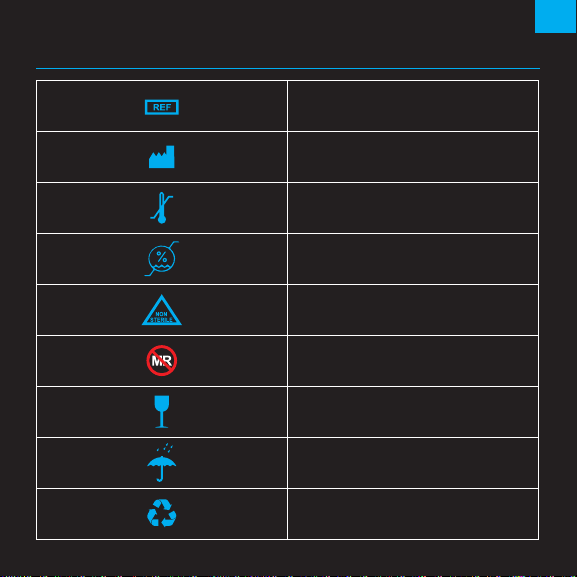
7
EN
SAFETYWARNING
»Do not apply stimulation of this device in the following conditions:
• Contralaterally across the chest because the introduction of electrical current into the chest may
cause rhythm disturbances to the heart, which could be lethal;
• over open wounds or rashes, or over swollen, red, infected, or inflamed areas or skin eruptions
(e.g., phlebitis, thrombophlebitis, varicose veins);
• in the presence of electronic monitoring equipment (e.g., cardiac monitors, ECG alarms);
• on children or incapacitated persons.
»Be aware of the following:
• consult with your physician before using this device;
• this device is not effective for pain associated with Central Pain Syndromes, such as headaches;
• this device is not a substitute for pain medications and other pain management therapies;
• this device is a symptomatic treatment and, as such, suppresses the sensation of pain that
would otherwise serve as a protective mechanism;
• stop using the device if the device does not provide pain relief;
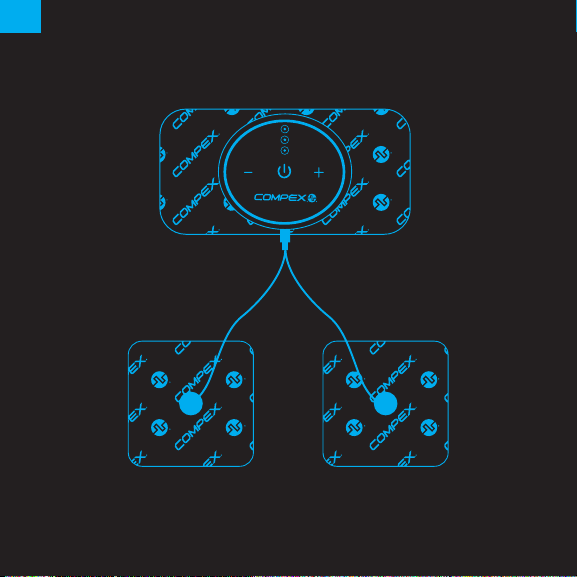
• use this device only with the electrodes, and accessories recommended for use by the
manufacturer.
»Store the device away from high-temperature and direct-sunlight. Storage outside of stated
storage temperature may result in measurement error or device malfunction.
»Do not share the use of the electrodes with others; electrodes are intended for single person use.
»Do not use the device while it’s charging.
»This device contains a lithium battery. If overheating of the device occurred during the charging,
stop the charging or operation immediately and contact customer support.
»Dispose of this battery-containing device according to the local, state, or federal laws.
»Skin burns may occur, and check the skin under the electrode periodically.