Dentsply Maillefer EASYPOST A0009 Manual

Maille fe r Instr ume n ts H oldin g S.à.r.l.
et ses filiale s (Trading S.à.r.l., Manufacturing S.à.r.l., Consulting S.à.r.l.)
Chemin du Verger 3, CH – 1338 Ballalgues Tél +41 (0)21 843 92 92 in o@dentsplymaille er.com
Switzerland Fax +41 (0)21 843 92 93 www.den ts plymail l efer.c om
DFU Easypost - F19 02 29.X/ 12 / 2001 - updated 09/2013
1/5
Product:
EASYPOST
REF A0009 - C0601
Ref doc.: F19 02 29.X/ 12 / 2001 - updated 09/2013
Este manual de utilização está também disponível, quando requisitado, nas seguintes línguas:
Português (PO ), Holandês (NL), Dinamarquês (DK), Sueco (S), Finlandês (FIN), Grego (G ),
Polacos (POL)
Deze gebruiksaanwijzing is, op aanvraag, eveneens verkrijgbaar in de volgende talen: Portugees
(PO ), Nederlands (NL), Deens (DK), Zweeds (S), Fins (FIN), Grieks (G ), Pools (POL)
Käyttöohje on saatavana myös se
uraavilla kielillä: Portugali
(PO )
, hollanti
(NL)
, tanska
(DK)
, ruotsi
(S), Suomi (FIN), Kreikka (G ), Puola (POL)
Denna bruksanvisning finns även att tillgå på följande språk: Portugisiska (PO ), Holländska (NL),
Danska (DK), Svenska (S), Finska (FIN), Grekiska (G ), Polska (POL)
Denne brugsanvisning kan også rekvireres på følgende sprog: Portugisisk (PO ), Hollandsk (NL),
Dansk (DK), Svensk (S), Finsk (FIN) græsk (G ) Polsk (POL)
Αυτή
η
οδηγία
είναι
επίσης
διαθέσι
µ
η
,
κατόπιν
ζήτησης
,
στις
ακόλουθες
γλώσσες
:
Πορτογαλέζικη
(PO ), Oλλaνδική (NL), ∆ανέζικη (DK), Σουηδική (S), Φιλανδέζικη (FIN), Ελληνική (G ) Πολωνική
(POL)
Ta instrukcja obsługi jest również dostępna, na zamówienie,
w następujących wersjach językowych:
Portugalski (PO ), Holenderski (NL), Duński (DK), Szwedzki (S), Fiński (FIN), Grecki (G ), Polski
(POL)

Maille fe r Instr ume n ts H oldin g S.à.r.l.
et ses filiale s (Trading S.à.r.l., Manufacturing S.à.r.l., Consulting S.à.r.l.)
Chemin du Verger 3, CH – 1338 Ballalgues Tél +41 (0)21 843 92 92 in o@dentsplymaille er.com
Switzerland Fax +41 (0)21 843 92 93 www.den ts plymail l efer.c om
DFU Easypost - F19 02 29.X/ 12 / 2001 - updated 09/2013
2/5
FOR DENTAL USE ONLY
DIRECTIONS FOR USE
EASY POST
A0009 - C0601
0) COMPOSITION
60% Zirconium enriched Glass Fiber, 40% epoxy resin.
1) INDICATION FOR USE
These products have to be used only in hospital environments; clinics or dental offices by qualified
dental personnel.
Application for the product:
In case of insufficient residual tooth substance (< 4 mm), the EasyPost is needed to support the
coronal restoration.
2) CONTRAINDICATIONS
Insufficient residual dentin: at least 2 mm of tooth structure is required around the preparation.
3) WARNINGS
None known
4) Precautions
•The Post is a Single Use device.
•Re-use of Easypost can increase the risk of cross contamination, poor cementing and breakage
•The post must be sterilized before insertion in the canal.
•Avoid touching the posts with your fingers after cleaning.
•Shortening the post should be done outside of the mouth.
•Dentsply Maillefer recommends the use of a rubber dam.
5) Adverse Reactions
In the present technical state, no adverse reaction has been reported so far.
6) Step-by-step instructions
1. Root canal preparation. In case of insufficient residual tooth substance (< 4 mm), the EasyPost
is needed to support the coronal restoration.
2. Predrilling with a Largo drill, Ref. A 0009 no. 1 or no. 2, depending on the diameter (rotation
speed 800-1200 rpm).
3. Precision drilling with the calibrating drill, Ref. C 0601 no. 1, no. 2, no. 3, or no. 4, depending on
the case (1000-2000 rpm).
4. Rinse and dry the canal.
5. Check positioning in mouth. Please keep a dentine collar of at least 2 mm around the
preparation.
6. Draw the post outside the oral cavity using a diamond-tipped disc.
7. Clean and condition the dentine walls with EDTA during 1 minute.
8. Rinse with sodium hypochlorite then dry with air (multifunctional syringe) and large diameter
paper points.
9. Clean the post with alcohol.
10. Adhere the tenon (silane does not need to be applied to the post):
DENTSPLY Maillefer recommend using a compomer or composite luting cement such as
Dyract Cem in conjunction with Prime&Bond + Self Cure.
The post as well as the space and access cavity must be treated with Prime&Bond + Self Cure.
Please follow the manufacturer’s instructions. The compomer or composite luting cement can
be applied in the canal using a rotary paste filler (Lentulo®).

Maille fe r Instr ume n ts H oldin g S.à.r.l.
et ses filiale s (Trading S.à.r.l., Manufacturing S.à.r.l., Consulting S.à.r.l.)
Chemin du Verger 3, CH – 1338 Ballalgues Tél +41 (0)21 843 92 92 in o@dentsplymaille er.com
Switzerland Fax +41 (0)21 843 92 93 www.den ts plymail l efer.c om
DFU Easypost - F19 02 29.X/ 12 / 2001 - updated 09/2013
3/5
11. Insert the post in the canal: please apply some pressure to ensure maximum adhesion.
12. Remove any excess.
13. Crown section: reconstruct a prosthetic false stump. We recommend using a composite core
buildup material (e.g. Dentsply Core X™).
7) DISINFECTION, CLEANING AND STERILIZATION:
Procedure for Posts and accessories
Foreword
For hygiene and sanitary safety purposes, all instruments not marked “sterile” must be cleaned, disinfected and
sterilized before usage to prevent any contamination.
Area of application
Disinfection and sterilisation before usage concerning:
Implantable devices:
Dentinal and radicular posts made of steel, titanium and glass fibers. Supports, kits and organiser systems for
posts.
General recommendation
1 - Use only a disinfecting solution which is approved for its efficacy (VAH/DGHM-listing, CE marking, FDA
approval) and in accordance with the DFU of the disinfecting solution manufacturer. For all metal instruments, it
is recommended to use anticorrosion disinfecting and cleaning agents
2 - For your own safety, please wear personal protective equipment (gloves, glasses, mask).
3 - The user is responsible for the sterility of the product as well as for the usage of damaged or dirty
instruments where applicable after sterility.
4 - Single use marked instruments are not approved for re-use.
5 - The water quality has to be convenient to the local regulations especially for the last rinsing step or with a
washer-disinfector.
6 - Plastic supports, are degraded by Hydrogen Peroxide (H
2
O
2
) solution.
7 – Supports made of aluminium are degraded in presence of caustic soda solutions with mercury salt. Do not
use acid (pH < 6) or alkaline (pH > 8) solutions.
8 - The washer-disinfector is not recommended for support made of aluminium
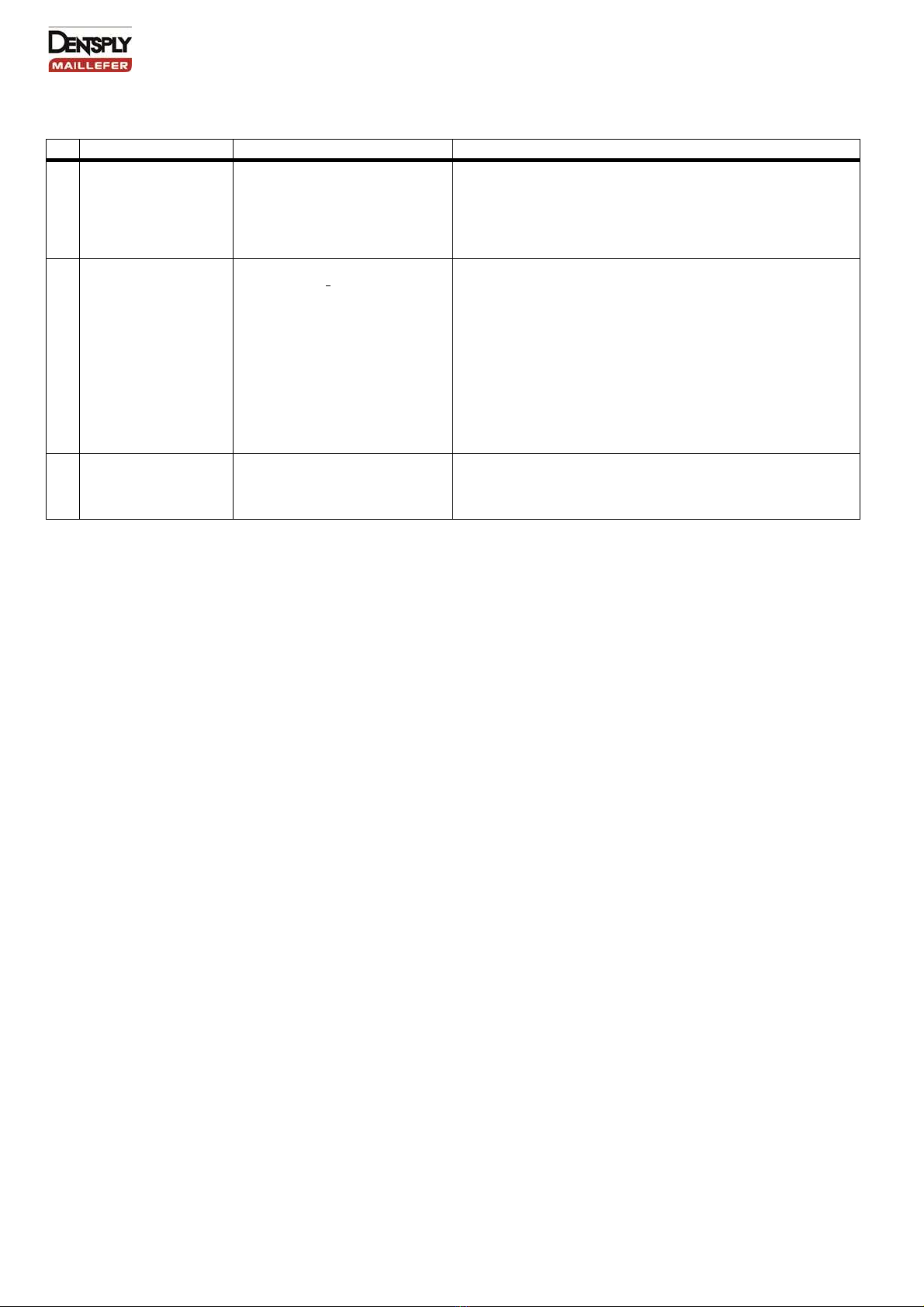
Step-by-step procedure
Operation Operating mode Warning
1a. Automated Cleaning
with washer-disinfector - Pl
ace the devices in a kit, support
or container to avoid any contact
between devices.
- Put them in the washer-
disinfector
(Ao value > 3000 or, at least 5 min
at 90 °C).
- Discard any posts with large obvious defects (broken, bent).
- Avoid any contact between devices when placing in the washer-
disinfector use kits, supports or container.
- Follow instructions and observe concentrations given by the
manufacturer (see also general recommendations).
- Use only approved washer-disinfector according to EN ISO
15883, maintain and calibrate it regularly.
OR
1b Manual Cleaning or
assisted by an
ultrasonic device
-
Place the devices in a kit, support
or container to avoid any contact
between devices.
- Immerse in the disinfecting
solution with cleaning properties,
assisted by an ultrasonic device if
suitable.
- No visible impurities should be observed on the Posts.
-
Discard any Posts with large obvious defects (broken, bent, and
twisted).
- Follow instructions and observe concentrations and time given
by the manufacturer (see also general recommendations).
- The disinfecting solution should be aldehyde free and without
di- or triethanolamines as corrosion inhibitor.
5. Rinsing - Abundant rinsing (at least 1 min) - Use quality water in accordance with local regulations.
- If a disinfecting solution contains a corrosion inhibitor, it is
recommended to rinse the posts just before the autoclaving.
- Dry on a single use non-weaved cloth, or with a drying machine
or filtered compressed air.
6. Inspection - Inspect devices and sort out
those with defects.
- Assemble the devices (stops)
- Dirty posts must be cleaned and disinfected again.
- Discard Posts which show any deformations (bent, twisted),
damages (broken, corroded) or defects (loss of colour coding or
marking) affecting the resistance, the safety or the performance
of the posts.
Maille fe r Instr ume n ts H oldin g S.à.r.l.
et ses filiale s (Trading S.à.r.l., Manufacturing S.à.r.l., Consulting S.à.r.l.)
Chemin du Verger 3, CH – 1338 Ballalgues Tél +41 (0)21 843 92 92 in o@dentsplymaille er.com
Switzerland Fax +41 (0)21 843 92 93 www.den ts plymail l efer.c om
DFU Easypost - F19 02 29.X/ 12 / 2001 - updated 09/2013
4/5
Operation Operating mode Warning
7. Packaging -
Place the devices in a kit, support
or container to avoid any contact
between devices and pack the
devices in “Sterilisation pouches”.
-
Avoid any contact between devices during sterilization. Use kits,
supports or containers.
- Check the validity period of the pouch given by the
manufacturer to determine the shelf life.
- Use packaging which are resistant up to a temperature of
141°C (286°F) and in accordance with EN ISO 11607.
8. Sterilization - Steam sterilisation at:
134 °C / 273°F during 18 min.
- The posts and the plastic supports must be sterilized according
to the packaging labelling.
- Use only autoclaves that are matching the requirements of EN
13060, EN 285.
- Respect the maintenance procedure of the autoclave device
given by the manufacturer.
- Use only this recommended sterilization procedure, validated
according to ISO 17665-1 standard.
- Control the efficiency (packaging integrity, no humidity, colour
change of sterilisation indicators, physico-chemical integrators,
digital records of cycles parameters).
- Ensure Traceability of procedure records
9. Storage - Keep devices in sterilization
packaging in a dry and clean
environment
-
Sterility cannot be guaranteed if packaging is open, damaged or
wet.
- Check the packaging and the medical devices before using
them (packaging integrity, no humidity and validity period).

Maille fe r Instr ume n ts H oldin g S.à.r.l.
et ses filiale s (Trading S.à.r.l., Manufacturing S.à.r.l., Consulting S.à.r.l.)
Chemin du Verger 3, CH – 1338 Ballalgues Tél +41 (0)21 843 92 92 in o@dentsplymaille er.com
Switzerland Fax +41 (0)21 843 92 93 www.den ts plymail l efer.c om
DFU Easypost - F19 02 29.X/ 12 / 2001 - updated 09/2013
5/5
Symbols
Manufacture date
Manufacturer
Caution : See directions for use
Recording on an input medium
Can be sterilized at the specified temperature
One use only
Opened packages are not replaced
Batch number
Reference number
Assortment
Fiberglass
Stainless steel
Keep away from sunlight and heat
Visit our website: www.dentsplymaillefer.com
Manufacturer:
Maillefer instruments Holding Sarl
Chemin du Verger 3
CH – 1338 Ballaigues
Switzerland
This manual suits for next models
1
Table of contents
Other Dentsply Maillefer Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual













