Dentsply Maillefer X-SMART IQ User manual

User Manual
EN

Table of contents
F1902142.EN / 05 / 2015 / updated 02/2016 3/74
Table of contents
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
1 Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2 Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
3 Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
4 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
5 Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
6 Step by Step Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
6.1 Document Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
6.2 DENTSPLY ENDO IQ™ Application Compatibility . . . . . . . . . . . . . 16
6.3 Package Contents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
6.4 Components not included . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
6.5 System Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
6.5.1 Turning on the iPad®and setting the Volume . . . . . . . . . . . . . . . . . . . . . . . 18
6.5.2 Activating the AssistiveTouch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
6.5.3 Activating the WiFi . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
6.5.4 Activating the Bluetooth® . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .21
6.5.5 Installing and Updating the DENTSPLY ENDO IQ™ Application . . . . . . . . . 22
6.5.5.1 Firmware update . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
6.5.6 Setting up the Complete System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
6.6 Motor Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
6.6.1 Description of the Motor Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
6.6.1.1 Description of the LED Colors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
6.6.1.2 The Auto Reverse (AutoRev) Functionality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
6.6.1.3 Description of the Sounds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
6.6.1.4 Stand-by Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
6.6.1.5 Battery Care . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
6.6.2 Inserting and removing the File . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
6.6.2.1 Inserting the File . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
6.6.2.2 Removing the File . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31

Table of contents
4/74 F1902142.EN / 05 / 2015 / updated 02/2016
6.6.3 Stand-alone mode: operation without the
DENTSPLY ENDO IQ™ Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .31
6.7 DENTSPLY ENDO IQ™Application . . . . . . . . . . . . . . . . . . . . . . . . .32
6.7.1 Starting the Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
6.7.2 Editing or creating a User Profile . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
6.7.3 Connecting the Motor Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .34
6.7.3.1 Connecting a Second Motor Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
6.7.3.2 Disconnecting the Motor Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
6.7.4 Interface Presentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38
6.7.5 Three Modes of Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .40
6.7.5.1 Motor Handpiece Information and Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
6.7.5.2 Start Treatment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
6.7.5.3 Start and Record Treatment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
6.7.6 Treatment Reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
6.7.6.1 Treatment Details . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
6.7.6.2 Treatment Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
6.7.7 Customize File Sequences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
6.7.7.1 Create new File . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
6.7.7.2 Create File Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
6.8 Storing and charging the Motor Handpiece . . . . . . . . . . . . . . . . . . . 51
7 Cleaning, Disinfection and Sterilization . . . . . . . . . . . . . . . . . . . . 52
7.1 Foreword . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .52
7.2 Single Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .52
7.3 Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
7.4 Cleaning, Disinfection and Sterilization of the Contra-Angle . . . . . .55
7.4.1 Pre-Treatment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .56
7.4.2 Manual Reprocessing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .57
7.4.3 Machine-based Reprocessing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .58
7.4.4 Lubrication of the Contra-Angle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .59
7.4.5 Sterilization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61
7.4.5.1 Approved Sterilization Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62

Table of contents
F1902142.EN / 05 / 2015 / updated 02/2016 5/74
8 Technical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
9 Motor Handpiece Error Code . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
10 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
11 Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
11.1 Exclusion of Liability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
12 Disposal of the Product . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
13 Identification of Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
13.1 Normative Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72

6/74 F1902142.EN / 05 / 2015 / updated 02/2016
FOR DENTAL USE ONLY
Introduction
Congratulations on your purchase of the X-SMART IQ™ endo motor.
The User Manual is always kept up to date by Maillefer Instruments
Holding Sàrl (hereafter Dentsply Maillefer) to bring it in line with the
latest developments. You will find the current version on
www.dentsplymaillefer.com and integrated into the DENTSPLY ENDO
IQ™ application.
In countries where the legal situation enables us to do so, we have
decided not to produce a printed version of the User Manual, for
environmental reasons.
If no printed version is enclosed in your national language and you wish
to have a printed one, we will be happy to send you a copy (free of
charge within 7 calendar days to any address in the EU). To order,
The User Manual is available in other languages on request.
This User Manual has been compiled with the utmost care.
Nevertheless, it is not always possible to completely rule out the risk of
error, despite all efforts todo so. We would appreciate yourfeedback in
this area. If any errors are noted, please contact Dentsply Maillefer.
DentsplyMailleferreservesthe righttochange the informationand data
contained in the User Manual without prior notice.
Maillefer Instruments Holding Sàrl
Chemin du Verger 3
CH-1338 Ballaigues
Switzerland
Telephone +41 21 843 92 92
Fax +41 21 843 92 93
www.dentsplymaillefer.com

Contraindications
8/74 F1902142.EN / 05 / 2015 / updated 02/2016
2 Contraindications
Read the following contraindications before use.
• In cases where a patient has been fitted with an implanted heart
pacemaker (or other electrical equipment) and has been cautioned
against the use of small electrical appliances (such as electric
shavers, hair dryers, etc.), X-SMART IQ™ must not be used.
• Do not use X-SMART IQ™ for implants or any other dental
procedure outside endodontics.
• Safety and effectiveness have not been established in pregnant
women and children.
• Clinical judgment needs to be applied by the end user of the device.

Warnings
F1902142.EN / 05 / 2015 / updated 02/2016 9/74
3 Warnings
Read the following warnings before use.
Dentist
• The motor handpiece is intended for endodontic treatment and may
only be used by trained and qualified professionals such as dentists
Ambient conditions
• The device must not be placed in humid surroundings or where it
can come into contact with liquids.
• Do not expose the device to direct or indirect heat sources. The
device must be operated and stored in a safe environment.
• The device can be operated up to a maximum temperature of 35°C
(95°F) and up to an altitude of 2 000 m (~ 6561ft 8in) above sea
level.
• Do not use the device in the presence of free oxygen, anesthetics
or flammable substances. The device must be operated and stored
in a safe environment.
• The device can cause radio interference or disrupt the operation of
equipment in the vicinity. If this happens, the interference should be
reduced by reorienting or repositioning the device or by screening
off the immediate environment. The electromagnetic radiations
emitted by the X-SMART IQ™ motor handpiece are below the
recommended limits specified by the applicable relevant provisions
(DIN EN 60601-1-2:2007).
• The device requires special precautions with regard to
electromagnetic compatibility (EMC) and must be installed and
operated in strict compliance with the EMC Directive. In particular,
do not use the device in the vicinity of fluorescent lamps, radio
transmitters, remote controls or portable or mobile RF
communication devices, even if they meet CISPR 8 requirements.
•TheiPad
®generates, uses and can radiate radio-frequency energy.
The use of the iPad®in a medical environment requires particular
care in regards to electromagnetic interference with other devices.
Please refer to the Apple User Manual for more information about
the iPad®’s electromagnetic compatibility.

Warnings
10/74 F1902142.EN / 05 / 2015 / updated 02/2016
•TheiPad
®protective case and the motor handpiece cover contain
permanent magnets. Magnets couldaffect the normal functioning of
pacemakers, implanted heart defibrillators and hearing aid devices.
Do not place the accessories near these devices or any other object
sensitive to static magnetic fields.
• Do not charge, operate or store at high temperatures. Comply with
the specified operating and storage conditions.
During treatment
• Gloves and a rubber dam are absolutely essential during treatment.
• If any irregularities with the device should occur during treatment,
switch it off. Contact Dentsply Maillefer or your Dentsply Maillefer
authorized service partner.
Battery
• Always use the original charger with the charging cable to charge
the device. Use of non-original chargers jeopardizes the safety of
the patient and user.
• If liquid should emerge from the motor handpiece, the batteries may
be leaking. In this event, stop using the device immediately and
contact Dentsply Maillefer or your Dentsply Maillefer authorized
service partner.
• Never open the device yourself. Opening the device voids the
warranty. Contact Dentsply Maillefer or your Dentsply Maillefer
authorized service partner.
• A device with a faulty battery must not be sent by air freight.
• Always check to ensure that both the motor handpiece and the
iPad®have a sufficient battery charge before starting treatment.
Contra-angle
• Never press the contra-angle pushbutton when the motor
handpiece is running or if it is coming to a stop. This will lead to
detachment of the instrument or cause the pushbutton to overheat.
• Never remove the contra-angle from the motor handpiece during
operation.
• Only use undamaged root canal instruments. Please refer to the
information provided by the manufacturer.

Warnings
F1902142.EN / 05 / 2015 / updated 02/2016 11/74
• Only insert the instrument when the contra-angle is stationary.
• Never place your fingers on the moving parts of the instrument
while it is running or coming to a stop.
• Before treatment, check the contra-angle for any damage or loose
parts.
• Only use the original Dentsply Maillefer contra-angle.
Root canal instruments
• Before use, make sure the instrument is securely locked in place.
• Never use continuous rotary instruments in reciprocating mode.
• Never use reciprocating instruments in rotary mode.
• Use the torque and speed settings recommended by the instrument
manufacturer.
iPad®, iPad®protective case and motor handpiece support/cover
• Hygiene measures to prevent cross-contamination are absolutely
essential. Dentsply Maillefer recommends using the iPad®
protective case as indicated in 6.5.6 Setting up the Complete
System or any other hygienic sleeve/barrier for medical
applications.
• The operating conditions and limitations specified by Apple®must
be complied with.
• Ensure the iPad®and the accessories are in a stable position
during the treatment.
• The motor handpiece cover can be magnetically attached to the
iPad®protective case. The device can fall if subjected to abrupt
movements.
• Do not disable the iPad®sound when using X-SMART IQ™.
• Do not disinfect the iPad®protective case if the protective plugs are
not properly inserted.
•Apple
®, the Apple logo and iPad®are trademarks of Apple Inc.,
registered in the U.S. and other countries. App Store®is a service
mark of Apple Inc.

Warnings
12/74 F1902142.EN / 05 / 2015 / updated 02/2016
DENTSPLY ENDO IQ™ application
• Dentsply Maillefer cannot be held liable for the developed
application if unauthorized modifications have been made (e.g.
jailbreaking, …).
• Treatment notes linked to patients in the application are subject to
the requirements of the applicable legislation on data privacy.
• User must take appropriate measures to protect any data
associated with a patient such as activating the device master
password, the encryption of the parameters for backup on iTunes®
and avoiding using the iPad®outside the medical environment.
• It is strongly recommended to periodically transfer all treatment
notes associated with a patient to your Dental Practice
Management Software and back-up your iPad®using iTunes®.
•DENTSPLY ENDO IQ™ does not replace your Dental Practice
Management Software.
• It is strongly recommended that all open applications are closed
before launching the DENTSPLY ENDO IQ™ application.
Accessories
• The use of the handpiece sleeve is mandatory. This product is
designed for single use and has to be disposed of and changed
after every treatment.
• Only use Dentsply Maillefer components/accessories and spare
parts.
• Using other makes of accessories/spare parts can lead to
increased emission of electromagnetic interferences or to reduced
electromagnetic interference immunity.
Repairs and Defects
• Do not use the device if you suspect damage or a defect.
• Repairs, alterations and modifications to the device are not
permitted unless the manufacturer's prior consent has been
obtained. Dentsply Maillefer cannot be held liable if the device has
been altered or modified. If a defect should occur, contact Dentsply
Maillefer or your Dentsply Maillefer authorized service partner.
Warnings
F1902142.EN / 05 / 2015 / updated 02/2016 13/74
Transport
• Intact devices can be transported by land freight or air freight in the
original packaging. The applicable requirements must be met (see
table below).
• Defective devices can also be transported by air freight or land
freight in the original packaging. If the battery is defective, the
device must not be transported by air freight under any
circumstances.
• Leaking liquid can be an indicator of a defective battery.
Standards and regulations that apply to the transport of X-SMART IQ™
Air freight Land freight
Intact device
or defective
device with
intact battery.
• UN 3481 Lithium batteries in
equipment.
• IATA Packing instruction 967
Part II.
• Special regulations issued
by airlines and national
regulations must be
complied with.
• UN 3481 Lithium batteries in
equipment.
• ADR Special provisions 188
f) and g).
Device with
defective
battery. Not possible.
• International, multilateral
agreements M 228 and M
259.
• ADR SV 661 (international,
road).
• Regulations issued by GRS
(German Joint Battery Take-
back System Foundation) for
the transport of waste lithium
batteries (FRG, road).

Precautions
14/74 F1902142.EN / 05 / 2015 / updated 02/2016
4 Precautions
Read the following precautions before use.
• Please check the compatibility of your DENTSPLY ENDO IQ™
application before updating the iPad®operating system.
• Always install the latest version of the DENTSPLY ENDO IQ™
application. Regularly check for updates on the App Store®.
Step by Step Instructions
16/74 F1902142.EN / 05 / 2015 / updated 02/2016
6 Step by Step Instructions
Refer to the chapter 3 Warnings to verify any special care to exercise
before starting to use the complete device.
Before use, please check the exact contents of the package. See 6.3
Package Contents.
6.1 Document Symbols
6.2 DENTSPLY ENDO IQ™ Application Compatibility
Symbol Identification
Consult instructions for use
If the instructions are not followed properly, operation may have risks for
the product or the user/patient
Additional information, explanation on operation and performance
Suggestion or advice
Specification Description
iPad®requirement
•iPad
Mini™
•iPad
Mini™ 2
•iPad
Mini™ 3
•iPad
Mini™ 4
Operating System •iOS8.x
•iOS9.x
Step by Step Instructions
F1902142.EN / 05 / 2015 / updated 02/2016 17/74
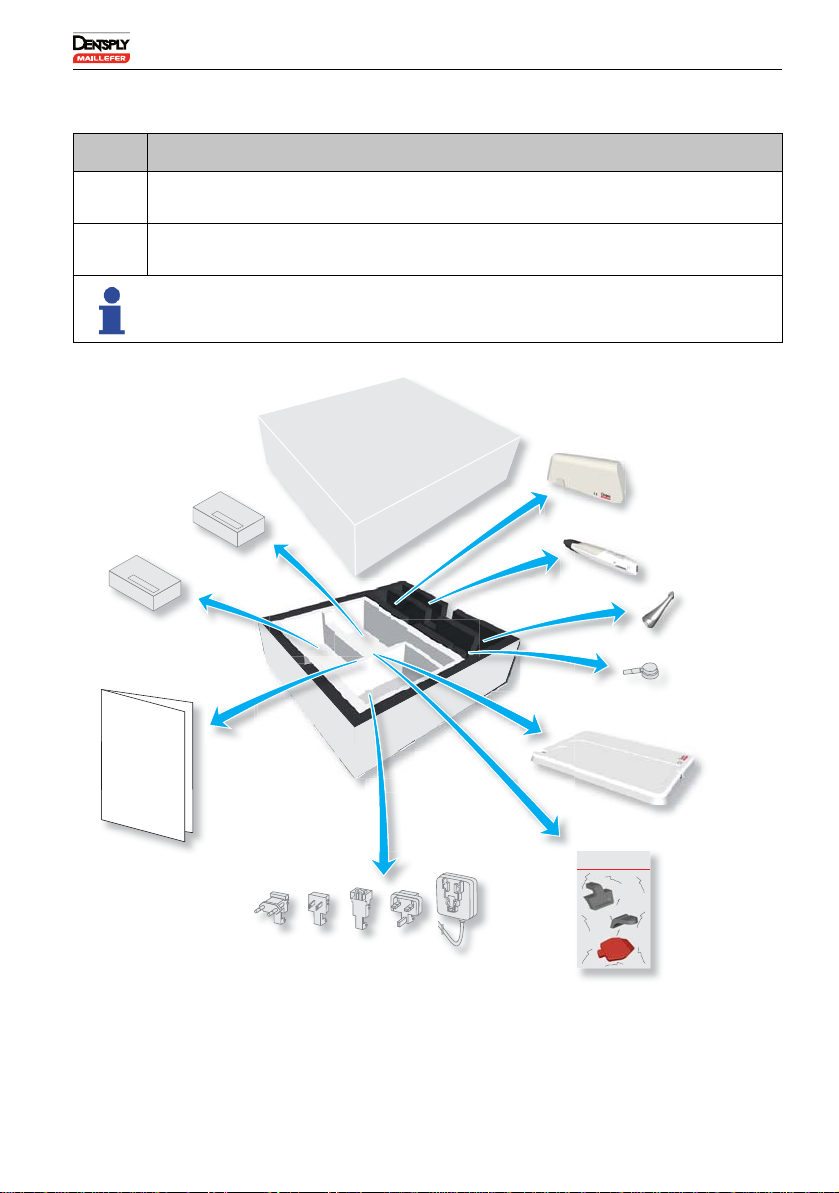
6.3 Package Contents
.
N° Action
ACarefully remove the device and the accessories from their packaging and
place them on a flat surface.
BCheck that the X-SMART IQ™ is supplied with all the components listed
below.
Not all components presented below are available in all kits.
Files
Files
Geing Started Guide
Justa few images to help you set up your motor easily
andget the most out of it.
Spray nozzle
(used for lubrication)
AC adapter
iPad Mini™
protective
case*
Motor handpiece
Contra-angle
Getting Started Guide
Rubber plugs and protective
case opener*
Motor handpiece support
and case
Files*
Samples of motor handpiece single use sleeve are included in the kits.
*These components are not available in all kits.
Step by Step Instructions
18/74 F1902142.EN / 05 / 2015 / updated 02/2016
6.4 Components not included
The following components are not included in the kit:
6.5 System Setup
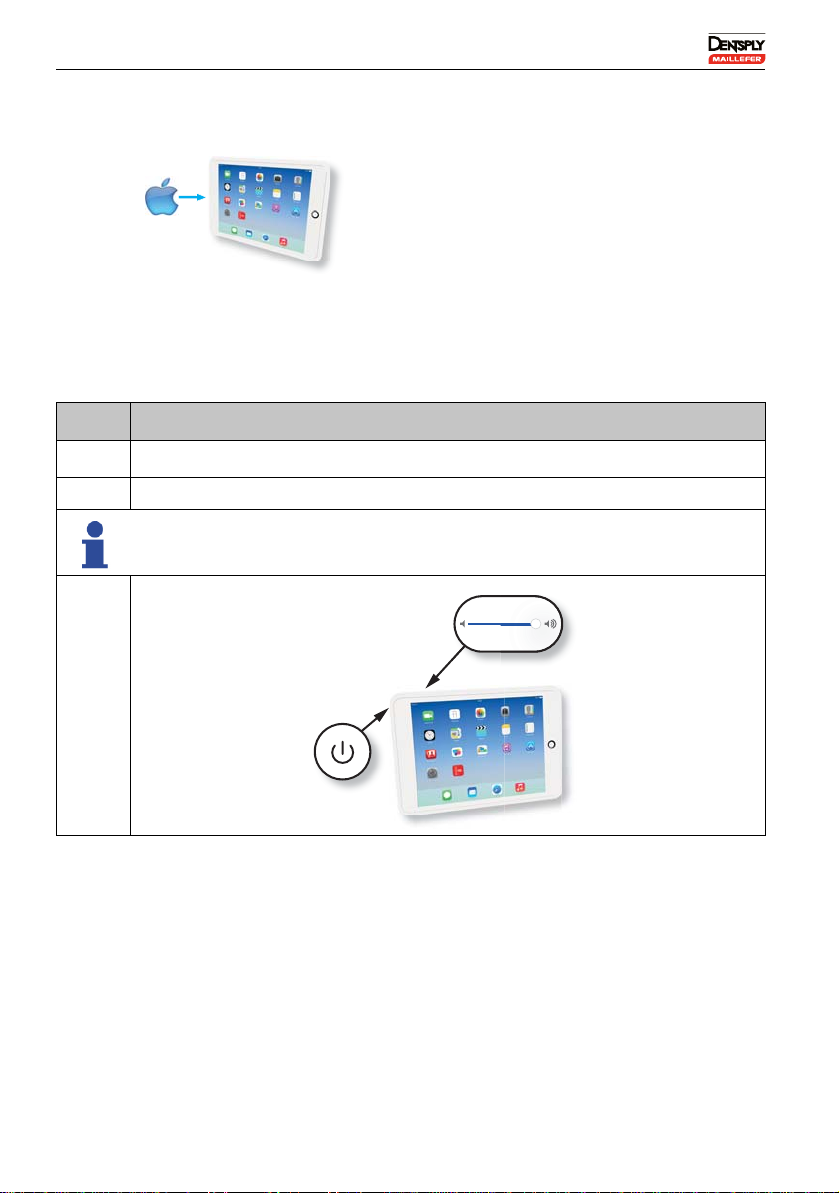
6.5.1 Turning on the iPad®and setting the Volume
iPad®with iOS app (not part of the kit).
N° Action
ATurn on the iPad®.
BAdjust the volume to maximum.
The application uses sounds to give general information, see 6.6.1.3
Description of the Sounds.
Step by Step Instructions
F1902142.EN / 05 / 2015 / updated 02/2016 19/74
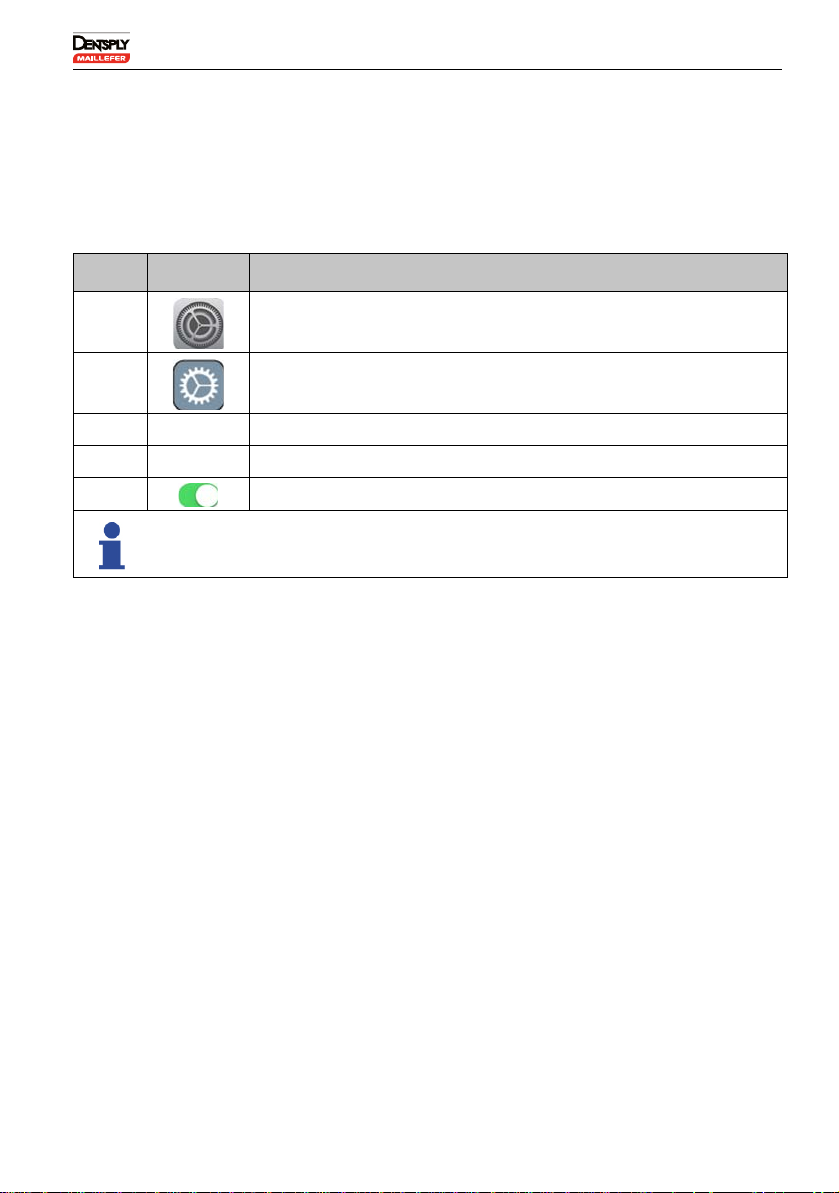
6.5.2 Activating the AssistiveTouch
The AssistiveTouch feature replaces the actions of the iPad®Home
button which is not accessible when in the protective case.
To activate the AssistiveTouch
N° Key Action
ASelect Settings.
BSelect General.
CSelect Accessibility.
DSelect AssistiveTouch.
ESet AssistiveTouch to ON.
A virtual "Home" key is displayed.
Table of contents
Other Dentsply Maillefer Medical Equipment manuals
Popular Medical Equipment manuals by other brands
de smit medical
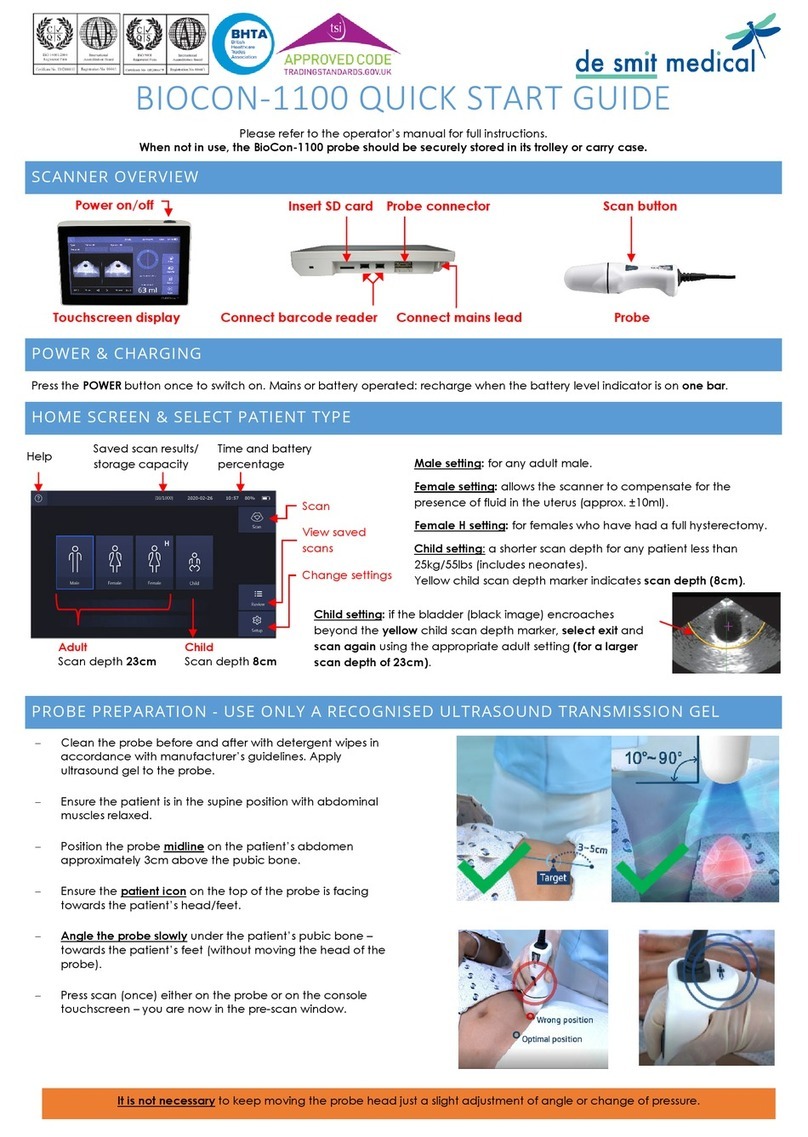
de smit medical CUBEscan BioCon-1100 quick start guide
VRI
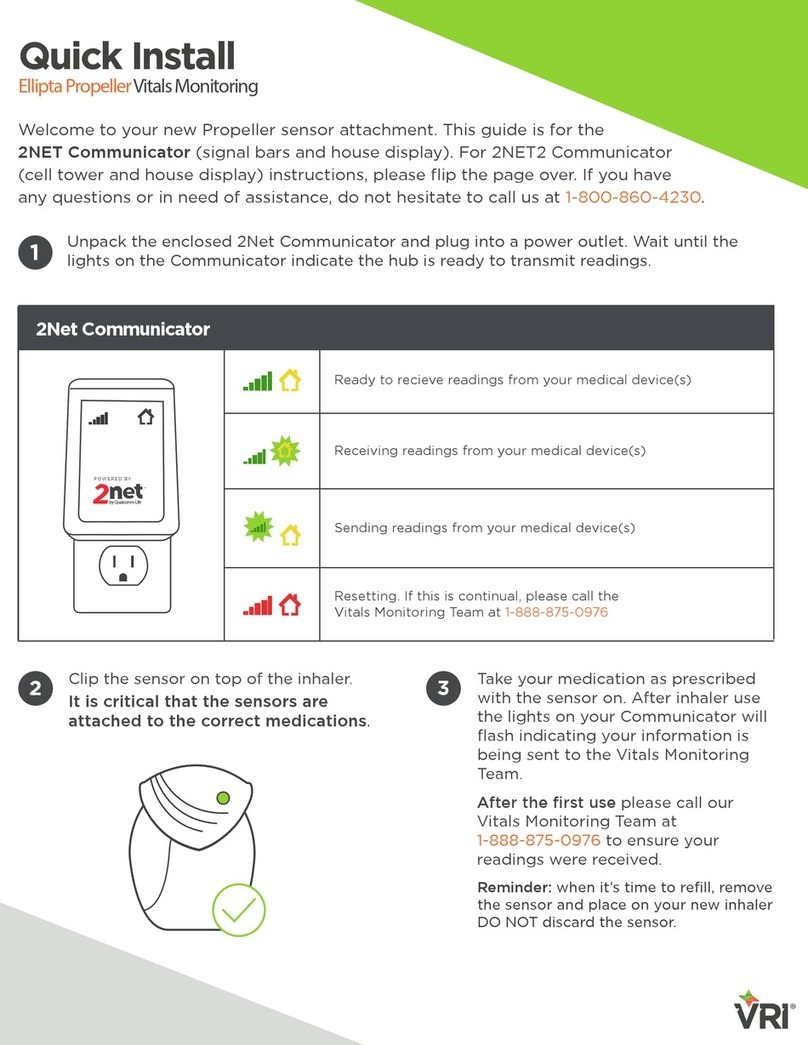
VRI 2NET Communicator Quick install
Chiesi
Chiesi Clenil Modulite user guide
GREINER
GREINER MULTILINE NEXT DC instruction manual

Cobi Rehab
Cobi Rehab Bariatric Comfort Foam Mattress user manual

B. Braun
B. Braun Aesculap Neurosurgery Instructions for use/Technical description