Diagnostic S-500 User manual

S-500
Automatic upper arm
blood pressure and pulse monitor
INSTRUCTION MANUAL
0197
5125
EN

CONTENTS
1. Safety information ......................................2
1.1 Warning ..........................................2
1.2 Precautions .......................................3
2. Product features .......................................4
3. Before taking readings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
3.1 Batteries .........................................5
3.2 Power supply (optional)..............................6
3.3 Settings ..........................................6
4. Taking a reading .......................................7
4.1 Important information ...............................7
4.2 Adjusting the cuff. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.3 Body posture during measurement . . . . . . . . . . . . . . . . . . . . . 8
4.4 Taking a reading . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
4.5 Memory .........................................10
5. Error messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6. Troubleshooting .......................................12
7. Specifications.........................................13
8. Important information regarding electromagnetic compatibility ...13
9. Blood pressure information ..............................16
9.1 What is blood pressure? ............................16
9.2 What is high blood pressure? . . . . . . . . . . . . . . . . . . . . . . . . 17
9.3 What is morning hypertension
(sudden morning surge in blood pressure)? . . . . . . . . . . . . . 18
Explanation of symbols19
Warranty card ..........................................20
1

1. SAFETY INFORMATION
1.1 Warning
Self-diagnosis and undertaking treatment based on the results of
measurements may be dangerous. Follow the advice of your doctor
or qualified healthcare professional.
People suffering from arrhythmia, diabetes, circulation problems or
medical conditions associated with stroke should use the device in
accordance with the recommendations of the doctor.
If the cuff filling continues, remove the cuff or turn off the power of
the device, otherwise it can lead to a hazardous situation.
This device is not intended for newborns, infants, and persons who
cannot communicate or take actions.
Do not use the pressure monitor for purposes other than
measurement of blood pressure in humans.
The measurements must be carried out exclusively using the cuff
or power adapter supplied by the manufacturer. Otherwise,
measurement results may be incorrect.
Do not use the pressure monitor near strong electrostatic charges
or electromagnetic fields, and do not use a mobile phone during the
measurement.
Do not use the pressure monitor together with hyperbaric oxygen
therapy or in the environment, where combustible gas may be
produced.
Do not use the device in the following locations:
- places where vibrations are present, i.e. in ambulances or
emergency helicopters.
- places where gas or flames are present.
- places where water or steam are present.
- places where chemicals are stored.
- places where the device may easily fall.
General arrhythmia, including premature atrial contractions,
premature ventricular contractions and atrial fibrillation may cause
inaccurate readings or errors.
The measurements and results must take into account environment
variables, otherwise this may cause incorrect measurements.
When replacing the power adapter or batteries, the person taking
the measurement should not touch those elements and the patient
simultaneously.
2

The batteries have a positive / negative polarity. If the battery does
not connect well with the device, do not try to insert it forcibly.
Do not use the Luer-type lock. If Luer-type lock connectors are
used in the construction of the tubes, there is a risk of accidentally
connecting to an intravascular flow system, allowing the pumping of
air into the blood vessels.
1.2 PRECAUTIONS
Do not disassemble, repair or make changes to the pressure
monitor or cuff.
Avoid high temperatures, moisture, dust and direct sunlight.
The casing of the device should be cleaned with a soft cloth
dampened in medical alcohol 75%.
Do not soak or clean the cuff with water.
After measurement, the cuff should be cleaned with a soft dry cloth.
Do not use in extreme high temperatures, high humidity or at high
altitudes. Use the device only in acceptable ambient conditions.
Do not place heavy objects on the power adapter or the device on
the cord.
Do not connect and disconnect the power adapter with wet hands.
Do not drop the device or expose it to strong shocks.
Do not use the device near large equipment that uses a relay for
ON/OFF type power switch.
If the device is not going to be used for an extended period of time,
remove the batteries.
Research on neonates, infants and pregnant women has not been
carried out. Do not use the measuring device on neonates, infants
and pregnant women.
The blood pressure monitor has been subjected to numerous
inspections to ensure measurement accuracy. The user should
perform the annual inspection and calibration of the device
recommended by the manufacturer.
Blood pressure measurements made by the device are equivalent
to those made by a qualified person using ausculatory method of
measurement with a cuff/stethoscope.
3
Keep the device out of reach of infants, young children and people
who are not able to give informed consent.
This product is suitable for blood pressure measurement at home
and for use by authorized medical personnel at hospital.
WARNING!Read the attached user manual.
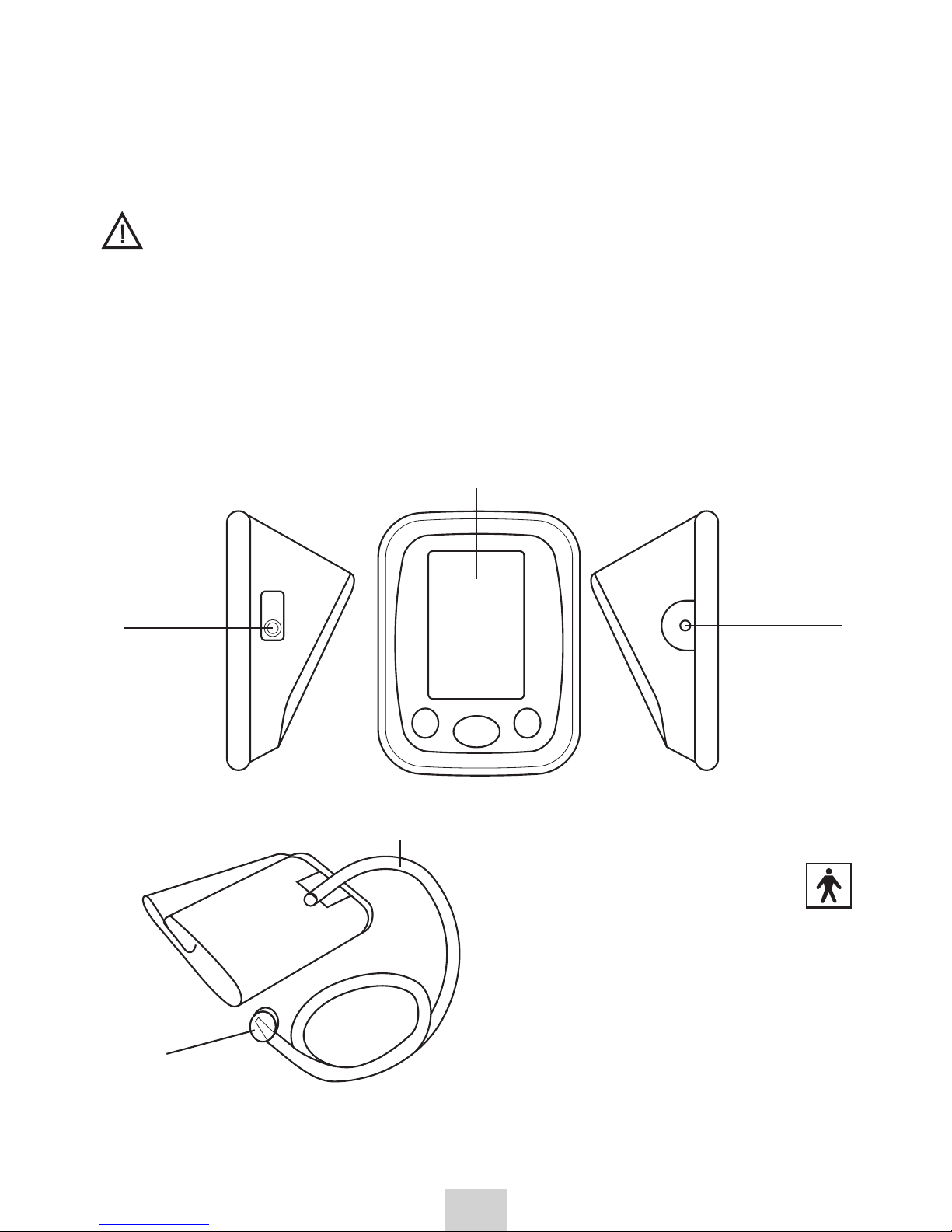
2. PRODUCT FEATURES
Indication for use: measuring blood pressure and pulse rate in adults at
home or for use by trained medical personnel at hospital.
Housing
Power
adaptera
LCD Display
Air
socket
Cuff (applied part
type BF)
Arm circumference:
220 to 360 mm
air
tube
Cuff
plug connecting
the air tube with
the blood pressure monitor
(insert into the air tube socket)
4
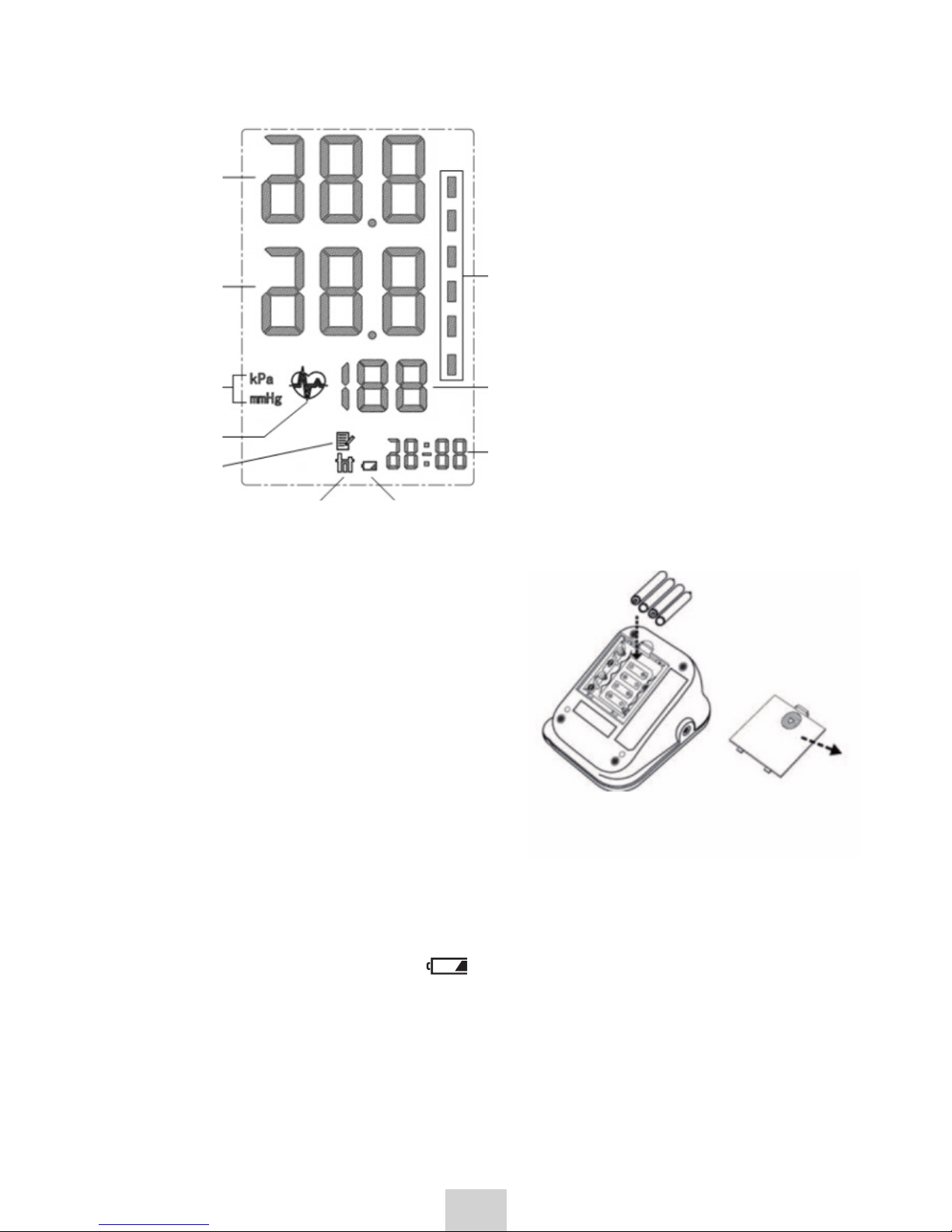
DISPLAY
Systolic
pressure
Designation
of units pulse
Low battery
symbol
Average
symbol
Memory
symbol
Heart symbol
Diastolic
pressure
time
Blood pressure
classification indicator
according to the World
Health Organization (WHO)
3. BEFORE TAKING READINGS
3.1 Batteries
3.1.1 Installation and replacement
1. Remove the battery cover.
2. Insert 4 standard AA alkaline
batteries as indicated
in the picture.
Use batteries of the same brand. Note that all the batteries are properly
installed, observing polarity.
3. Reinstall the battery cover.
4. Batteries must be replaced when the icon indicating low battery level
appears.
If the low battery symbol is displayed, they should be replaced
with new ones, otherwise the device will not operate properly.
Use 4 alkaline batteries 1.5 V AA of the same brand.
Do not mix old and new batteries.
If the device is not going to be used for an extended period of time,
remove the batteries.
After replacing batteries, you must reset the time and date.
5
3.1.2 Battery life
Four new batteries LR6 (AA) are sufficient for approx. 200
measurements, if the measurements will be taken once a day at
room temperature (23°C).
The batteries included in the kit will be only useful for
demonstration purposes. These batteries will probably not be
enough to complete 200 measurements.
You can check the battery status in the lower right corner of the
screen. If the low battery symbol is displayed, battery power is low
and they should be replaced with new ones.
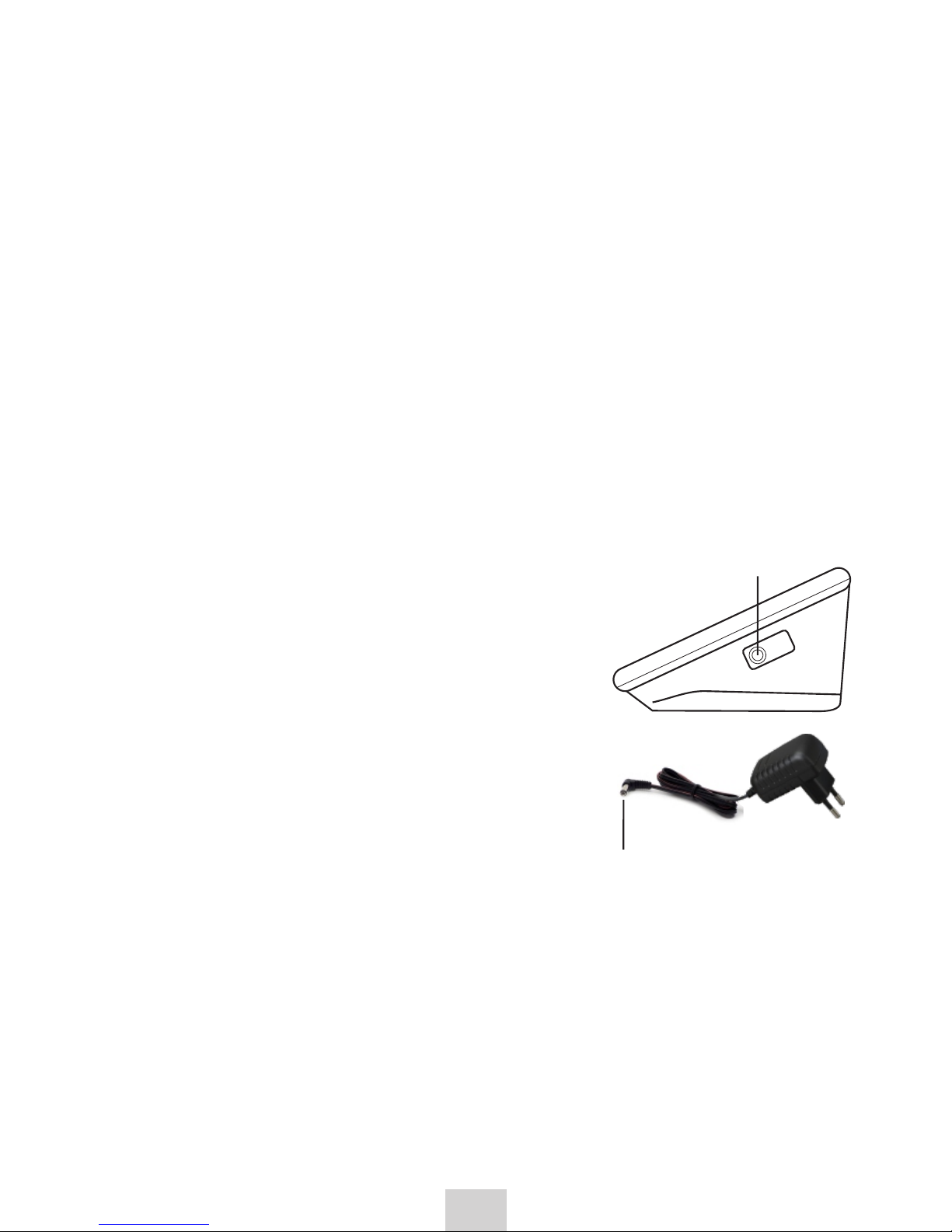
3.2 Power adapter (optional)
1. Connect the power supply plug into the power supply socket (right side
of the device).
2. Plug the power adapter unit into electrical outlet.
Use power adapter suitable for local mains
voltage (mains current 100~240 V)
Specification of power adapter:
input: 100-240 V, 50/60 Hz, 6V,
min. 400mA;
We recommend using only the power
adapter supplied by the manufacturer,
model Diagnostic ZID 6-1 (100~240 V,
50/60Hz, 6 V, 1000 mA (1 A))
If the device is defective, unplug the
power supply or the power cor.
Do not touch the power adapter with a wet
hand.
Do not tangle the wires during usage.
The power adapter is added to the set
optionally (charged additionally)
3.3 Settings
With the device powered off:
1. Press the SET button, the appliance enters the date setting mode.
a) Changing values
press the MEM button to go one digit up
if you press and hold the MEM button, the value will change
quickl
Air
plug
Power
supply
6

b) Set the two digits indicating year.
c) Press the SET button and continue to set the values for month
d) Repeat steps a) to c) to set the month, day, hour and minutes
2. Przeliczanie jednostek (mmHg
na kPa)
a) Pressing the MEM button,
automatically changes the unit
as shown.
b) Dokończyć ustawianie, wcisnąć
przycisk ON/OFF aby wyjść.
4 TAKING MEASUREMENT
4.1
4.1 Important information
For a minimum of 30 minutes before the measurement of blood
pressure, you should not eat, drink alcohol, smoke, take a shower,
or exercise. Do not take any medication that can raise blood
pressure.
Blood pressure measurements should not be taken in a state of
nervousness or anxiety. When we are nervous, worried or agitated
our blood pressure increases.
Relax for 5-10 minutes before measurement. Sit
in a comfortable and relaxed body position. When measuring blood
pressure do not move and talk. Feet should be placed in one
position, breathe freely and calmly.
The cuff used to measure blood pressure should cover
approximately ¾ of the arm. It should be easy to wrap.
If possible, measurements should always be taken on the same
arm.
Measuring blood pressure at the same time on different days
should give similar results (except in the case of external factors,
such as for instance exercise).
Change of medication or dietary supplement can affect the results
of measurement. Before starting or stopping taking medication or
supplement, you should consult with your doctor
7

4.2 Adjusting the cuff
1. Insert the air tube in the socket on the left side of the device.
2. Insert the end of the cuff under the metal buckle, with the velcro facing
out.
3. Wrap the cuff approximately 2-3 cm above the elbow. For best results,
wrap the cuff on bare skin, at heart level.
4. The compression of arm caused by tucked up sleeve may prevent
accurate reading.
5. After wrapping the cuff, make sure that there is sufficient space under
the cuff to fit a finger.
6. If the cuff does not fit on the arm, the accuracy of measurements may
be incorrect.
Do not fold the cuff or the air tube.
To disconnect the cuff, remove the air tube plug from the device.
Measurement can be started only after wrapping the cuff properly.
The cuff must be replaced if there is a leak or when the cuff is not
operating properly.
In order to ensure the proper accuracy of readings, you should only
use the cuff supplied by the manufacturer.
4.3 Body posture during measurement
Relax, rest the elbow on the table with palm
facing up; the cuff should be
at heart level. Accuracy of readings
may be reduced if the cuff is not wrapped
properly. The arm should be at the same height
as the heart. If the arm is too low, the reading
results will be too high. If the arm is too high, the
reading results will be too low
Air tube should be
placed in the middle of
the arm.
Palm
facing up
The arm should
be at the same
height
as the heart
8
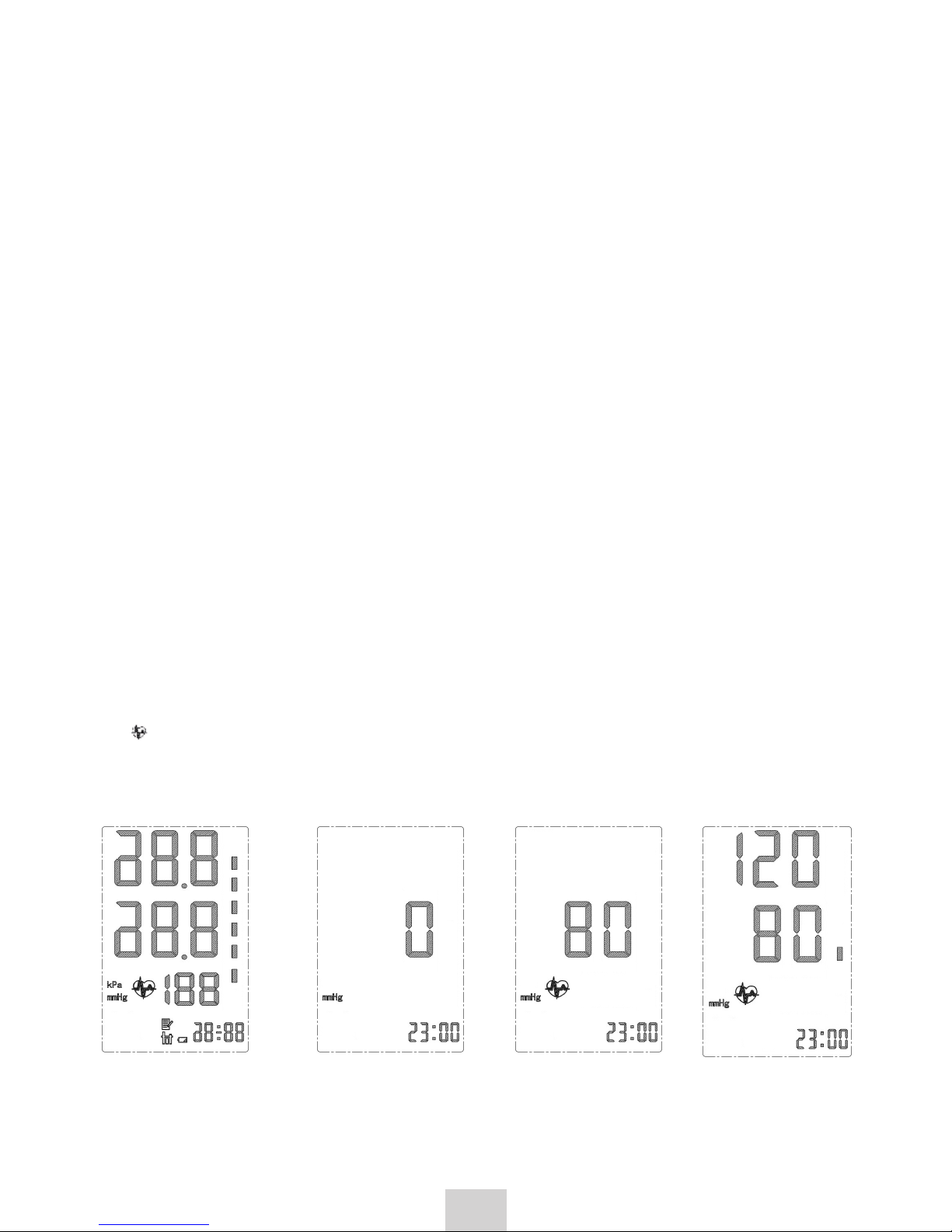
4.4 Taking measurement
After installing the batteries and wrapping the cuff, the device is ready to
take measurement:
1. To ensure that the results are most accurate, during taking the reading,
relax, do not smoke, do not take deep breaths, do not talk loudly and try
not to move.
2. Press the [ON/OFF] button; the display will light up for 1 second, as
shown in Figure 1.
3. Then the display will switch as shown in Figure 2.
4. When the device detects pulse, the heart symbol starts blinking, as
shown in Figure 3. The cuff is filled with air and the measurement of
pulse rate and blood pressure is performedi.
5. After the measurement, the air will automatically be released from the
cuff and the results of the measurement will be displayed on the screen
as shown in Figure 4. The bar on the right side of the display will
indicate the level of blood pressure. The classification and definition of
blood pressure level is presented on page 10.
6. You can turn off the device or compare the reading with previous results.
7. The device automatically turns off after 3 minutes.
8. If a problem occurs during the measurement, the screen displays the
"Err" (error) message.
9. At the end of the measurement, if irregular pulse is detected, the symbol
will appear on the screen .
Fig.1 Fig.2 Fig.3 Fig.4
9

Classification and definition of blood pressure level
Notes:
Do not perform self-diagnosis based on the obtained results. Follow
the advice of your doctor or qualified healthcare professional.
The bar on the right side of the display and the color of the element
indicate the level of blood pressure, blood pressure classification
and definition, as shown in the figure above.
If the device causes any discomfort during the measurement or
fails to work properly, it should be turned off and its use
discontinued.
Pressure reduction time with 260 mmHg (34.67 kPa) to 15mmHg
(2kPa) does not exceed 10 sec.
If the cuff is inflated to the level of 300 mmHg (40 kPa) and inflation
continues, disconnect the cuff from the unit or turn off the power.
4.5 Memory
Internal memory stores up to 90 measurement results.
1) Recalling results from memory
a) To access memory, press the MEM button.
b) The device displays the average result of 3 most recent
measurements.
c) After pressing the MEM button, the user can view data from the
newest to the oldest. Pressing the SET button allows to view the
results in reverse order.
d) If the heart symbol is displayed together with the data stored in the
memory , it indicates that irregular pulse rate was detected during
the measurement.
Red
Yellow
Green
Systolic ‡180 mmHg
and/or Diastolic ‡110 mmHg
Systolic between (160-179) mmHg
and/or Diastolic (100-109) mmHg
Systolic between (140-159) mmHg
and/or Diastolic (90-99) mmHg
Systolic between (130-139) mmHg
and/or Diastolic (85-89) mmHg
Systolic between (120-129) mmHg
and/or Diastolic (80-84) mmHg
Systolic < 120 mmHg and
Diastolic < 80 mmHg
10

Note: Holding the MEM button longer will erase all data from the memory.
2) Deleting data from memory:
a) You must go into memory mode.
b) Press and hold down the MEM button until “---” is displayed.
c) All data will be deleted, it is not possible to delete a single record.
d) Press the ON/OFF button to exit the memory mode and turn off
the device.
5. ERROR MESSAGES
List of error codes.
HOW TO REPAIRCAUSE
ERROR
CODE
1. Reconnect the air tube plug into the device.
2. Leakage of the cuff or tubing. Purchase a new cuff.
3. Make sure that the cuff is properly wrapped. Take another
measurement.
The cuff is too loose, make sure that the cuff is properly
wrapped.
Take another measurement.
The power is turned on, the cuff
fills too slowly or the device does
not connect to the cuff.
Er30
Weak signal or cuff is too loose
Er 2
Calculation error, strong impact,
error during installation or design
Remain motionless.
Take another measurement.
Remain motionless.
Take another measurement.
Please take another measurement.
Replace the batteries with new ones.
Er 3
Bad signal, movement or talking
during measurement
Er 5
Incorrect measurement.
Er 7
Battery level too low, not able to
inflate
Lo
11
6. TROUBLESHOOTING
In the event of irregularities during operation, please refer to the
following:
PROBLEM
After replacing batteries and
turning on the device, nothing
displays
The measured values are too
high or too low
Pumping is too slow or the cuff
is not inflated
The air from the cuff is released
too quickly.
Zmierzona wartość jest inna od
tej zmierzonej w szpitalu lub w
gabinecie lekarskim
HOW TO REPAIR
1. Check battery polarity.
2. If you still cannot turn on the device, reinstall or replace the
batteries.
1. Make sure that the cuff is correctly wrapped.
2. If the user's clothing restricts normal blood flow, it should be
removed and take another measurement.
3. Relax, rest the elbow on the table with palm facing up; the
cuff should be at heart level. Take another measurement.
1. Reconnect the air tube plug with the device.
2. Leakage of the cuff or air tubing.
Replace with a new one.
1. The cuff is too loose, make sure that the cuff is properly
wrapped.
1. The value of blood pressure varies throughout the day, as
well as is caused by the emotional and physical health
status
2. Take notes of the differences and consult them with your doctor.
12
7. SPECIFICATION
Description
Display
Measurement
range
Memory of
Power source
Protection against
electric shock
Operating
environment
Weight
Useful life of parts
Automatic upper arm blood
pressure monitor
Digital display LCD
Pressure: 0~280 mmHg
Pulse rate: 40~180 beats/min
90 measurements
4 AA alkaline batteries power or
power adapter DC 6.0 V,
min. 400 mA (optional)
Type BF
Temperature: +5°C ~ 40°C
Humidity: + 93%
Pressure: 70.0kPa~106.0kPa
Altitude: + 3000 m
280g (without batteries)
Casing 5 lat
or 10000 times
Cuff 10000 times
Power adapter 50000 times
Model Diagnostic S-500
Oscillometric method
Pressure: ±3mmHg (±0.4 kPa)
Pulse: ±5%
Idle for 3 minutes
Approximately 200 measurements
IP 21
Temperature: -25°C ~ 70°C
Humidity: 10 ~95%
Pressure:50 kPa~106 kPa
138 mm×110mm×68 mm
Cuff (Available arm circumference:
220mm to 360mm)
power adapter (optional)
4 x AA alkaline batteries
User manual (Warranty card)
Measurement
principle
Accuracy
Automatic power
off
Battery life
IP Classification
Storage and
transport
conditions
Dimensions
Content
This device is intended for use in a household environment. Specifications may change without prior
notice. Batteries must be disposed of in accordance with local regulations.
8. IMPORTANT INFORMATION CONCERNING
ELECTROMAGNETIC COMPATIBILITY (EMC)
In view of the increased number of electronic devices such as computers or mobile phones, medical
devices may be susceptible to electromagnetic interference from other devices. Electromagnetic
interference may result in the malfunctioning of medical equipment and create a potentially dangerous
situation.
In order to regulate the requirements for electromagnetic compatibility (EMC), with a view of preventing
dangerous situations associated with the product, the IEC60601-1-2 standard was introduced.
The standard specifies the level of immunity to electromagnetic interference as well as maximum levels
of electromagnetic emissions in relation to medical devices. This medical device meets the
requirements of the IEC60601-1-2:2007 standard, both for immunity and emissions.
However, you should take special care:
Do not use cellular phones or other devices that generate strong electrical or magnetic fields near
medical devices. This can result in abnormal operation of the device and create a potentially
dangerous situation. It is recommended to keep a distance of at least 7m. If the distance is shorter,
the correct operation of the device must be verified.
13

Guidelines and manufacturer's declaration - electromagnetic emissions
The devices are intended for use in the electromagnetic environment as described below.
The customer or the user of the device should assure that the device is used in such an environment.
Emission test
The emission of radio
frequency waves;
CISPR standard
The device uses radio-frequency energy only for its
internal functions.
Therefore, these emissions are very low and should not
cause interference in nearby electronic equipment.
Grupa 1
The emission of radio
frequency waves;
CISPR standard
The device can be used in all buildings, including
residential buildings, and those that are directly
connected to the public low-voltage network,
supplying power to buildings intended for residential
purposes.
Klasa B
Harmonic emissions
IEC 61000-3-2
non applicable
Voltage fluctuations/flicker
emissions IEC 61000-3-3
Fulfillment
of requirem
Guidelines regarding electromagnetic
environment
Guidelines and manufacturer's declaration regarding electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below. The customer or the user of the
DEVICE should assure that it is used in such an environment.
Immunity test
Electrostatic discharge
(ESD) IEC 61000-4-2
Fast transient/burst IEC
61000-4-4
Surges IEC
61000-4-5
Voltage dips, short
interruptions and
voltage changes on
power supply inlets
IEC 61000-4-11
Magnetic field of the
power supply frequency
(50/60 Hz)
Test level, IEC 60601
standard
± 6 kV contact
± 8 kV air
± 2 kV for power supply
lines
± 1 kV for input/output
± 1 kV differential mode
± 2 kV common mode
<5 % UT (>95 % dip in
UT) for 0.5 cycle
40 % UT (60 % dip in
UT) for 5 cycles
70 % UT (30 % dip in
UT) for 25 cycles
<5 % UT (>95 % dip de
UT) for 5 s
3 A/m
Compatibility
level
± 6 kV contact
± 8 kV air
Non applicable
Non applicable
Non applicable
3 A/m
Electromagnetic environment - guidelines
Floors should be wooden, concrete or
made of ceramic tiles. If floors are covered
with synthetic materials, the relative
humidity should be at least 30%. If ESD
interferes with the device, you should
consider the use of compensatory
elements i.e. wrist strap, grounding.
The quality of power supply should be
adequate for typical commercial installation
or hospital environment.
The quality of power supply should be
adequate for typical commercial installation
or hospital environment.
The quality of power supply should be
adequate for typical commercial installation
or hospital environment. If the user [of the
device or system] requires continuous use
even during power interruptions, it is
recommended to connect the device or
system to emergency power supply.
The level of magnetic fields of power
sources should be within the limits
applicable for typical commercial
installations or hospital environment.
14
15
Guidelines and manufacturer's declaration regarding electromagnetic immunity
With respect to the EQUIPMENT or SYSTEMS, which do not serve as LIFE
SUPPORTING SYSTEMS
GUIDELINES AND MANUFACTURER'S DECLARATION REGARDING ELECTROMAGNETIC IMMUNITY
The device is intended for use in the electromagnetic environment specified below. The customer or the user
of the DEVICE should assure that it is used in such an environment.
Immunity test Test level, IEC
60601 standard
Compatibility
level
Electromagnetic environment - guidelines
3V Portable and mobile radio communication
measures should be used at a distance from
any of the elements [of the DEVICE or
SYSTEM], including cables, which is not lower
than the recommended distance
calculated from the transmitter frequency
equation.
Recommended distance d = 1.2
d = 1.2 80 MHz to 800 MHz
d = 2.3 800 MHz to 2.5 GHz
where P is the maximum power rating of the
transmitter in watts (W) as specified by the
manufacturer, and (d) is the recommended
distance in meters (m).
Field strengths from fixed RF transmitters, as
determined in field measurements of
electromagnetic fields, should be lower than the
compatibility level for each frequency range.
Interference may occur in the vicinity of
equipment marked with the following symbol:
Recommended
distance
d = 1.2
3V/m
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Conducted radio-
frequency signal
IEC 61000-4-6
Emitted radio-
frequency signal
IEC 61000-4-3

Recommended spacing between portable and mobile radio communication
equipment and the DEVICE
The [DEVICE or SYSTEM] is intended for use in the electromagnetic environment in which the interference caused
by the emission of radio waves is controlled. The buyer or the user of the [DEVICE or SYSTEM] can help prevent
electromagnetic interference by keeping a minimum distance between portable and mobile radio communication
equipment (transmitters) and the [DEVICE or SYSTEM], as recommended below, according to the maximum
output power of the communication equipment
For transmitters assessed at the maximum output power not listed below, the recommended distance d in meters
(m) can be estimated using the equation corresponding to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts according to the transmitter manufacturer.
NOTE 1 at 80 MHz and 800 MHz, the distance for the higher frequency range applies.
NOTE 2: these guidelines do not apply in all situations. The propagation of electromagnetic waves is affected by
the absorption and reflection from the buildings, objects and people.
Recommended spacing between portable and mobile radio
communication equipment and the DEVICE in relation to DEVICES that
do not serve as LIFE SUPPORTING SYSTEMS
Maximum rated power
of the transmitter
W
0 01
0 1
1
10
100
Distance according to frequency of the transmitter m
150 kHz do 80 MHz
d = 1,16
0 12
0 38
1 2
3 8
12
80 MHz do 800 MHz
d = 1,16
0 12
0 38
1 2
3 8
12
800 MHz do2.5 GHz
d = 2,33
0 23
0 73
2 3
7 3
23
9.BLOOD PRESSURE INFORMATION
9.1 What is blood pressure?
Blood pressure (BP) is the pressure exerted by the circulating blood on
blood vessel walls and is one of the essential vital parameters.
During blood pressure measurements two values are read:
Systolic blood pressure is a measure of the pressure during
contraction of the heart
Diastolic blood pressure is a measure of the pressure during
relaxation of the heart
16

9.2 What is high blood pressure?
High blood pressure also known as HBP or hypertension is a commonly
misunderstood medical condition. It is believed that people with
hypertension are tense, nervous or hyperactive, but pressure has nothing
to do with personality. A person can in fact be peaceful, relaxed and at the
same time suffer from hypertension. Let's look at the facts regarding blood
pressure, so that we gain a better understanding on how the body works
and why it is prudent to start taking care of yourself right now, regardless of
what the results of our blood pressure are.
By maintaining blood pressure in the "healthy" range we achieve the following:
The risk of overloading and damaging the walls of blood vessels is
reduced
The risk that the heart will have to pump blood with greater force to
overcome obstacles is reduced
We protect our body through regular supply of oxygen-rich blood to
the tissues
In accordance with the standard of the World Health Organization (WHO)
classification of blood pressure is as follows:
Category Systolic (mmHg) Diastolic (mmHg)
Indicated <120 i <80
Normal 120-129 i/lub 80-84
Mild hypertension 130-139 i/lub 85-89
Hypertension ≥140 i/lub ≥90
Hypertension type 1 140-159 i/lub 90-99
Hypertension type 2 160-179 i/lub 100-109
hypertensive crisis ≥180 i/lub ≥110
These categories have been defined by the American Heart Association.
This table applies to adults aged 20 and older.
17

9.3 What is morning hypertension (sudden morning
surge in blood pressure)?
Morning high blood pressure or a surge in blood pressure in the morning is
referred to as the average of weekly morning blood pressure reading,
measured 1 to 2 hours after the waking up, the value of which exceeds 135/85
mm Hg. Studies indicate that excessive morning blood pressure poses risk of
cardiovascular incidents - including ischemic and haemorrhagic strokes. It has
also been demonstrated that cardiovascular incidents are more frequent in
the morning, combined with the morning surge in blood pressure. Of all the
days of the week, the incidents such as heart attack, stroke and heart failure
are particularly likely to occur on Mondays.
Also organ damage and diabetes complications, as well as microangiopathy
and heart attack in older people are associated with the morning rise in blood
pressure. Morning surge in blood pressure also is also linked to the initial
stages and the development of atherosclerosis. Patients who undergo regular
blood pressure checks may still experience a morning surge in blood pressure
and it is so in 50% of the cases. The risk of stroke in patients with high morning
blood pressure is about 78% higher compared with 48% of the risk in patients
suffering from hypertension, but who do not experience increase in blood
pressure in the morning. Morning hypertension is also related to changes in
the size and the rhythm of the heart. This can lead to a heart attack or heart
failure.
Morning surge may be detected only during 1-2 hours after waking up,
therefore, it is recommended to measure blood pressure at home.
Reference standards
IEC 60601-1:2005 Medical electrical equipment - Part 1: General safety
requirements and operation principles.
IEC 60601-1-2:2007 Medical electrical equipment - Part 1-2: General safety
requirements and operation principles - Collateral standard: Electromagnetic
compatibility - Requirements and tests
EN 1060-1:1995 + A2:2009 Non-invasive Sphygmomanometers - part 1: General
requirements
EN 1060-3:1997 + A2:2009 Non-invasive Sphygmomanometers - part 3: Additional
requirements for electromechanical blood pressure measuring systems
ANSI/AAMI SP-10:2002+A1:2003+A2: 2006/(R)2008 Hand, electronic or automatic
sphygmomanometers
ANSI/AAMI/ISO 81060-2-2009 Non-invasive sphygmomanometers - Part 2: Clinical
validation for automatic measurement type
18

EXPLANATION OF SYMBOLS
Degree of protection
Important warnings
IP21
Pause and turn on
Systolic pressure in mmHg
Diastolic pressure in mmHg
Pulse rate
Note on installing battery
Direct current
The product complies with the requirements of the European Union
Date of the last revision
Manufacturer
Manufacturing date
Serial number
Read the user manual
Batch number
Catalog number
Type BF: device, cuff and tubing are designed to provide special protection against electrical shocks.
START/STOP 0197
Rev.
The worn out product should be taken to a waste collection
facility. Contains components that are dangerous for the
environment. The correct disposal of the device allows to
preserve valuable resources and avoid negative effects on
health and the environment, which may be threatened by
inappropriate handling of waste.
Rev. 2016.09.13
przygotowana na podstawie polskiej wersji językowej z dnia 2015.10.27
an
Table of contents
Other Diagnostic Blood Pressure Monitor manuals