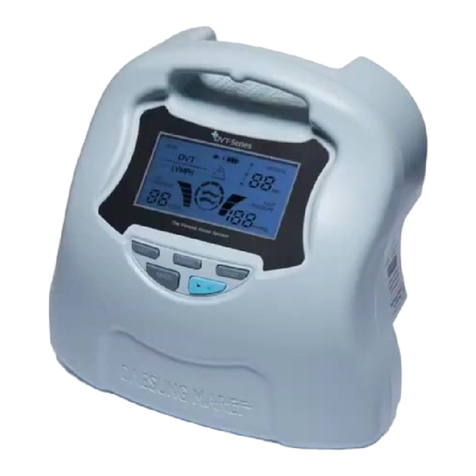
2-1. Operation of the device
- The device is for indoor use only. Do not also use the device in
high humidity places such as sauna, bathroom.
(This result in mechanical defect or physical damage caused by an
electric shock or scald.)
- When using or transporting this product please take care not to shake
or drop this device as it may cause the device to malfunction or fail to
operate.
- Do not insert many plugs in an outlet.
(Use an outlet that has a circuit breaker to prevent the risk of fire.)
- Do not plug in or plug out with wet hands, which may cause electric
shock.
- Do not turn on the power switch before applying sleeve to a patient.
Connect the tubings after the sleeve are applied to the patient. Turn the
power on to the device after connecting the tubings to the air socket at
rear of device to ensure self check.
- Do not operate with patient standing or sitting down. There could be a
risk of malfunction of the device and sleeve.
- The manufacturer recommends using basic pressure settings for initial
use. Caution is recommended for manual pressure settings (Leg : over
60mmHg, Foot : over 140mmHg)
- To avoid the risk of electric shock, use the power supply with protective
grounding.
(There is a risk of fire and electric shock.)
- Insert the plug completely in an outlet to prevent the risk of fire.
- Install the power plug in a place where it can be easily removed.
- Do not pull the wire to move the device.
(If the wire is damaged, it may cause a fire or electric shock during use.)
Information on Safety and Handling
2
10