EchoNous EchoNous Bladder User manual

P003948-007, Rev. A
March 2021
www.EchoNous.com
User Manual

INTENTIONALLY LEFT BLANK

EchoNous Bladder & Vein User Guide i
Contents
CHAPTER 1 Important Information 1
About the user guide 1
Manual conventions 1
Symbols in this user guide 2
Warnings, cautions and contraindications 2
Version information 2
Product description 2
Intended uses 3
Contraindications 3
System and transducer applications 4
Training 7
Package contents 7
System features 8
Classifications 8
Patient environment 9
Cautions, warnings, and contraindications 9
Warnings 10
Cautions 12
Contraindications 13
Labeling 14
Contact information 19
United States 19
European economic area 20
Australia 20
Trademarks and document copyright 20
CHAPTER 2 Getting Started 23
Unpacking the boxes 23
Options 23
Connecting and disconnecting the power supply 23
Setting up the system 24
Basic device control 24
System controls 25
System status icons 25
System notification icons 26
Menu 26
Settings 26
Network printer setup (optional) 27
Bluetooth printer setup (optional) 28

ii EchoNous Bladder & Vein User Guide
Barcode scanner setup (optional) 29
Imaging screens 29
Bladder volume screens 31
Vascular access screens 32
Recommended ultrasound transmission gel 34
Recommended ultrasound probe sterile sheaths 34
CHAPTER 3 Scanning 35
Preparing for the exam 35
Default settings 35
Bladder scanning 36
Measuring bladder volume 36
Bladder Measurement Correction 39
Audio feedback 39
Tips for obtaining bladder volume 40
Phantom mode 42
Abdominal Ultrasound imaging 42
Ultrasound presets 43
Ultrasound scanning 43
Ultrasound measurement 44
Ultrasound imaging movies 46
Vascular access 46
Vascular access scanning 47
Enabling depths of 4 and 5 CM 48
Vascular access measurement 48
Vascular access movies 49
Common functions 50
Multiple scans 50
Deleting images 50
Ending/saving exams 50
After use 50
Turning the system off 50
CHAPTER 4 Other Functions 51
Annotations 51
Text annotations 52
Voice annotations 52
Exam notes 53
Entering patient details 53
Printing 54
Export 55
Scan review 55
Deleting exams and patients 56
Data security 57

EchoNous Bladder & Vein User Guide iii
Setting a device password 57
CHAPTER 5 Maintenance and Troubleshooting 59
Maintenance 59
Battery maintenance 60
Probe cleaning and disinfection 60
Cleaning 61
Disinfection 61
Troubleshooting 63
Clinical troubleshooting 63
EchoNous customer support 64
CHAPTER 6 Connectivity and Accessories 65
Accessories 65
System connectivity 66
Power sources 66
Internal battery 66
Power supplies (chargers) 67
EchoNous stand setup 67
EchoNous AI Station power management 70
EchoNous AI Station troubleshooting 71
Cleaning 71
Disinfection 71
CHAPTER 7 Specifications 73
System dimensions 73
Environmental operating and storage conditions 74
Mode of Operation 74
Power supplies (chargers) 74
Internal battery 74
Measurement accuracy 75
Degree of protection against ingress of water 76
CHAPTER 8 Safety 79
Ergonomics 79
External materials 79
Disposal 79
Electrical safety 80
Electromagnetic compatibility (EMC) 81

iv EchoNous Bladder & Vein User Guide
Power supplies (chargers) 85
Biological safety 85
Acoustic output 85
CHAPTER 9 Advanced device and IT setup 87
Administrator setup 87
Software updates restrictions 87
User management 87
Multiple device setup 89
Imaging restrictions 89
Data recording restrictions 90
Storage access restrictions 90
CHAPTER 10 References 91
Glossary 92

EchoNous Bladder & Vein User Guide 1
CHAPTER 1 Important Information
About the user guide
This user guide is a reference tool for users of the EchoNous™ System; it does not constitute medical advice nor
provide clinical training, instruction in exam protocols, or information on how to interpret scans.
This guide should be read before the System is used. The System is intended to be used in a medical facility.
Manual conventions
The following style conventions are used in this manual:
•Buttons found on your EchoNous System are indicated in bold italics, such as Scan button. This style is also
used to describe areas of the display touch screen, such as Image name.
•“Tap” refers to touching the screen quickly with your finger.
•“Click” refers to pressing and releasing the button on the probe unit.
•“Drag” refers to touching the screen with your finger and then moving your finger across the screen.
•“Swipe” refers to moving your finger across the screen quickly.
•“Pinch” refers to moving two fingers in a pinch motion or pinch release motion across the screen.
•“Check” refers to tapping a check box to enable the associated function.
•“Uncheck” refers to tapping a check box to disable the associated function.
•“Select” refers to tapping a menu item from a menu list.
•New terms that describe functions are introduced in italics, such as exam.
•Numbered steps must be performed in a specific order.
•Bulleted items are lists in no specific order.
•Links to other sections within the manual appear colored and underlined, such as see Contact information.

Important Information
2EchoNous Bladder & Vein User Guide
Symbols in this user guide
Warnings, cautions and contraindications
•Warnings, cautions and contraindications are included throughout this manual along with the content to
which they apply. In addition all warnings and cautions are listed in the “Cautions, warnings, and
contraindications” on page 9.
•A contraindication is a specific situation in which the device should not be used because it may be harmful to
the patient.
Version information
This user guide applies to the EchoNous System with software version V5.0
Product description
The EchoNous System consists of a display running EchoNous System software, connected to one or two
ultrasound probes (EchoNous Bladder™ probe and/or EchoNous Vein™ probe) via cables. The System provides
portable ultrasound imaging in either handheld or AI Station scenarios. The System with its two probes has been
designed to support the following clinical applications:
•Noninvasive urological imaging
•General ultrasound imaging
•Vascular access procedures
The System generates and transmits ultrasound energy in the form of pulses in the 2 to 6 MHz range for the
EchoNous Bladder probes and in the 6 to 14 MHz range for the EchoNous Vein probe into a patient, detects the
reflected pulses, and processes the information to generate ultrasound images and measure anatomical
structures.
The EchoNous System display is an off-the-shelf Android tablet approved, pre-configured, and supplied by
EchoNous. The display comes with a power supply. When the display is connected to an ultrasound probe, the
combination is configured as a medical electrical System.
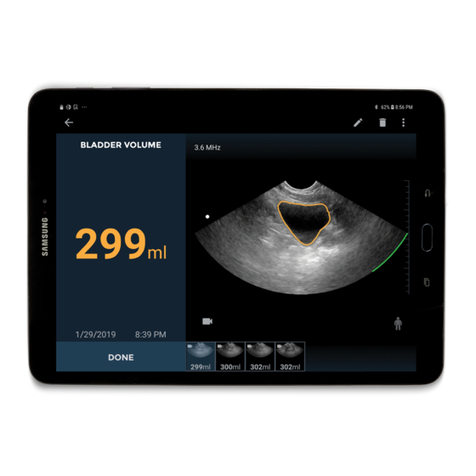
When equipped with the EchoNous Bladder probe, the System enables the user to automatically measure
bladder volume with or without a brightness mode (B-mode) image for guidance (FIGURE 1. System user
interface and display). The System processes data in real-time, and when the user is not scanning in the correct
location to detect a bladder, the display guides the user to the correct location, reducing measurement errors.
The user can elect to scan organs beside the bladder, including the kidney and prostate, with controls to change
brightness and depth in this mode.
Warning A warning describes precautions to prevent injury or loss of
life.
Caution A caution describes precautions to prevent damage to the
device.
Note A note provides supplemental information.

EchoNous Bladder & Vein User Guide 3
Intended uses
The System enables the user to perform Vascular Access procedures when connected to an EchoNous Vein
probe.
Images are able to be annotated by using voice or text. Examination notes can be entered.
The System provides optional wireless connectivity allowing remote storage and device tracking.
Accessories for wireless printing, barcode scanning, and the EchoNous AI Station are supported and available in
some countries.
FIGURE 1. System user interface and display
Intended uses
The EchoNous System is for noninvasive imaging of the human body and is intended for the following
applications: Abdominal, Musculoskeletal, Pediatric, Small Organ, and Peripheral vessel. Users must have
ultrasound training for abdominal, musculoskeletal, pediatric, small organ, and peripheral vessel imaging.
The System can also be used to obtain an image of the bladder that is used to automatically determine bladder
volume.
Contraindications
•The System is designed for transcutaneous scanning only. Do not attempt intracavitary imaging; in particular,
trans-esophageal, trans-vaginal and trans-rectal scans are contraindicated.
•The System is not intended for ophthalmic use or any use causing the acoustic beam to pass through the eye.
•Show care when scanning near a wound to avoid damaging or
further injuring the affected area.
•Review the ultrasound image when measuring bladder volume on
pregnant or postpartum patients, and adjust the bladder outline if it
has included amniotic fluid or the uterus in the measurement.
Federal (USA) law restricts this device to sale by or on the order of a
physician.

Important Information
4EchoNous Bladder & Vein User Guide
System and transducer applications
* Small organ use is prostate
TABLE 1. EchoNous System applications
Clinical Application Mode of Operation
General Specific B-mode
B-mode/
THI
Ophthalmic Ophthalmic
Fetal imaging
& other
Fetal
Abdominal
Intra-operative (specify)
Intra-operative (neuro)
Laparoscopic
Pediatric
Small organ (specify)*
Neonatal cephalic
Adult cephalic
Trans-rectal
Trans-vaginal
Trans-urethral
Trans-oesophageal (non-
cardiac)
Musculoskeletal (conventional)
Musculoskeletal (superficial)
Intravascular
Other (specify)
Cardiac Cardiac adult
Cardiac pediatric
Intravascular (cardiac)
Trans-esophageal (cardiac)
Intra-cardiac
Other (specify)
Peripheral
vessel
Peripheral vessel
Other (specify)

EchoNous Bladder & Vein User Guide 5
System and transducer applications
* Small organ use is prostate
TABLE 2. EchoNous Bladder applications
Clinical Application Mode of Operation
General Specific B-mode
B-mode/
THI
Ophthalmic Ophthalmic
Fetal imaging
& other
Fetal
Abdominal
Intra-operative (specify)
Intra-operative (neuro)
Laparoscopic
Pediatric
Small organ (specify)*
Neonatal cephalic
Adult cephalic
Trans-rectal
Trans-vaginal
Trans-urethral
Trans-esophageal (non-cardiac)
Musculoskeletal (conventional)
Musculoskeletal (superficial)
Intravascular
Other (specify)
Cardiac Cardiac adult
Cardiac pediatric
Intravascular (cardiac)
Trans-esophageal (cardiac)
Intra-cardiac
Other (specify)
Peripheral
vessel
Peripheral vessel
Other (specify)

Important Information
6EchoNous Bladder & Vein User Guide
TABLE 3. EchoNous Vein applications
Clinical Application Mode of Operation
General Specific B-mode
B-mode/
THI
Ophthalmic Ophthalmic
Fetal imaging
& other
Fetal
Abdominal
Intra-operative (specify)
Intra-operative (neuro)
Laparoscopic
Pediatric
Small organ (specify)*
Neonatal cephalic
Adult cephalic
Trans-rectal
Trans-vaginal
Trans-urethral
Trans-esophageal (non-cardiac)
Musculoskeletal (conventional)
Musculoskeletal (superficial)
Intravascular
Other (specify)
Cardiac Cardiac adult
Cardiac pediatric
Intravascular (cardiac)
Trans-esophageal (cardiac)
Intra-cardiac
Other (specify)
Peripheral
vessel
Peripheral vessel
Other (specify)

EchoNous Bladder & Vein User Guide 7
Training
Training
The EchoNous System is intended to be used by clinicians with appropriate professional qualifications and
clinical training.
All users should read the generic ALARA education program supplied with your EchoNous System (see enclosed
ISBN 1-93004 7-71-1, Medical Ultrasound Safety) or the Health Canada “Guidelines for the Safe Use of Diagnostic
Ultrasound” available on the Health Canada website. This program outlines the guiding principle for diagnostic
ultrasound, where the qualified user keeps ultrasound exposure to “as low as reasonably achievable” while
performing a diagnostic examination.
In addition to the above, users intending to use the ultrasound imaging function must have appropriate training
in ultrasound. Appropriate information on training may be obtained by contacting EchoNous or your local
professional body.
For users measuring bladder volume, an understanding of anatomy and location of the bladder is required.
Ultrasound training is required if bladder outline adjustments are to be performed.
Users performing vascular access procedures must have appropriate training in ultrasound guided vascular
access. Appropriate information on training may be obtained by contacting EchoNous or your local professional
body.
Package contents
The EchoNous Bladder™ carton contains the following:
•An EchoNous Bladder probe
•Three Quick Reference Guides: P005601, P005602, and P005613
•A USB Flash device containing:
— The EchoNous Bladder and Vein User Guide
— Training materials including:
•A generic ALARA education program (see enclosed ISBN 1-93004 7-71-1, Medical Ultrasound Safety)
•Terms and conditions of Warranty
•Quick Reference and Setup Guides
The EchoNous Vein™ carton contains the following:
•An EchoNous Vein probe with electronics box
•Quick Reference Guide
•You must have appropriate ultrasound training before using
the System for general ultrasound imaging or adjusting
bladder measurement outlines.
•You must have ultrasound guided vascular access training
before using the System for vascular access procedures.

Important Information
8EchoNous Bladder & Vein User Guide
•A USB Flash device containing:
— EchoNous Bladder and Vein User Guide
— Training materials including a generic ALARA education program (see enclosed ISBN 1-93004 7-71-1,
Medical Ultrasound Safety)
•Terms and conditions of Warranty
•Quick Reference and Setup Guides
The EchoNous Display carton contains the following:
•A tablet (or hand-held display) preconfigured with the EchoNous System software
•A Setup Guide
System features
The front of the EchoNous Samsung tablet display and probes are shown below.
FIGURE 2. System features
The camera for barcode scanning is on the rear of the display.
Classifications
•The System is internally battery powered during scanning.
•The EchoNous Type C Power Supply P005179 classification for Protection against electric shock: Class II
equipment.
TABLE 4. System features
1 Power on/off 4 USB port
2 Volume up/down 5 EchoNous Vein probe
orientation marker
3 Touch screen 6 EchoNous Bladder probe
orientation marker

EchoNous Bladder & Vein User Guide 9
Patient environment
•The EchoNous Bladder probe is Type BF Applied Part.
•The EchoNous Vein probe (P005134) is Type BF Applied Part.
•The EchoNous Bladder probe is classified as IPX4.
•The EchoNous Vein probe is classified as IPX4.
•The EchoNous System is not for use within an oxygen-rich environment.
Patient environment
The System is intended to be used in a medical facility. It is battery powered, and the user is expected to bring the
System into the patient environment for use. Power supply connections for recharging are to remain outside the
patient environment. See FIGURE 3. Patient environment for a drawing of the patient environment. When a
power supply is connected, ensure the connection can be easily disconnected.
FIGURE 3. Patient environment
If fluid is spilled on the probe cable or display, immediately remove the fluid with a soft dry cloth. Carefully
inspect the probe cable, USB connector, and display connectors for signs of fluid ingress. If there are any signs of
fluid ingress or if the device exhibits any unusual behavior, do not use, and contact EchoNous customer support
or your EchoNous distributor immediately. If required, also follow the cleaning and disinfection instructions (see
Cleaning).
Cautions, warnings, and contraindications
To ensure the device is not damaged and user and patient safety is maintained, please read and follow the
cautions and warnings below.
•Equipment not suitable for use in the presence of a FLAMMABLE
ANAESTHETIC MIXTURE WITH AIR OR WITH OXYGEN OR NITROUS
OXIDE.
•Do not use the System near high-frequency surgical equipment, as it
could create a burn hazard.
•Do not recharge the System in the patient environment.
Patient environment (side view) Patient environment (top view)
1.5m 1.5m

Important Information
10 EchoNous Bladder & Vein User Guide
Warnings
•Show care when scanning near a wound to avoid damaging or
further injuring the affected area.
•You must have appropriate ultrasound training before using the
System for general ultrasound imaging or adjusting bladder
measurement outlines.
•You must have appropriate ultrasound guided vascular access
training before using the System for vascular access procedures.
•Review the ultrasound image when measuring bladder volume on
pregnant or post-partum patients, and adjust the bladder outline if it
has included amniotic fluid or the uterus in the measurement.
•Equipment not suitable for use in the presence of a FLAMMABLE
ANAESTHETIC MIXTURE WITH AIR OR WITH OXYGEN OR NITROUS
OXIDE.
•Do not use the System near high-frequency surgical equipment, as it
could create a burn hazard.
•Do not recharge the System in the patient environment.
•Recharge the System only with the Power Supplies (chargers)
provided.
•The EchoNous Power Supplies are dedicated units to be used
exclusively with the EchoNous System only.
•Only connect the Power Supplies to a mains supply rated at 100-240V
and 50-60Hz.
•Do not use the device or Power Supply if there are signs of damage.
•Be aware of latex allergy. Some commercially available transducer
covers contain latex.
•Check the connecting cable, connectors, and System housings
before use for cracks or fraying. Do not use if damaged.
•No modification of this equipment is allowed.
•This device contains no user-serviceable parts. Please contact
EchoNous customer support or your EchoNous distributor for
maintenance or repair.
•Remove all particles and other matter from crevices and surfaces
when cleaning the System and components.
•The device is supplied unsterile.
•Clean and disinfect the patient-applied part between patients.
•Before cleaning or disinfection turn the System off and disconnect
from the power supply.
•Do not submerge the display or the power supply (charger) as electric
shock could result. The EchoNous Vein and EchoNous Bladder probes
may be immersed 12mm (1/2 inch) from the cable strain relief for high
level disinfection. The remainder of the probe is IPX4 which allows
water splashing onto the probe. The display is IPX0 and has no
protection against ingress of water.

EchoNous Bladder & Vein User Guide 11
Cautions, warnings, and contraindications
•Clean and disinfect the System before placing in a bag for transport.
Use the supplied EchoNous probe holder to store the probe. Clean
and disinfect the probe holder regularly.
•After cleaning or disinfection examine the ultrasound probe and
display as appropriate for cracks or leaks, and if damage exists
discontinue use of the System and contact EchoNous customer
support or your EchoNous distributor.
•The user must not touch any device connectors while in physical
contact with the patient.
•The ultrasound probes are connected to the EchoNous System
display running EchoNous System software to configure a medical
System. The display has been certified by EchoNous as part of a
medical System to EN IEC 60601-1: Edition 3.1.
•Do not connect the EchoNous System display to external computers
or peripherals using the USB port unless the System is outside the
patient area. Failure to comply with these guidelines may result in
electric shock.
•Mounting the EchoNous System display on the AI Station or MODO
Stand is configuring a medical System. Only use the EchoNous
provided AI Station or MODO Stand accessory (P005149 or P004013).
•Only connect accessories that are specified as being compatible with
the EchoNous System. Contact EchoNous customer support or
your EchoNous distributor for information on compatible accessories
and Systems.
•Do not open or modify the EchoNous Power Supply (P005179) or any
other supplied power supplies – Risk of electric shock
•Connecting electrical equipment to an (MSO) effectively leads to
creating a medical electrical System, and can result in a reduced level
of safety.
• MSOs (if provided with the medical electrical System) are to be used
only for supplying power to the tablet display and optional printer in
non-operating mode.
•Risk of shock or personal injury when connecting any equipment that
has not been supplied as a part of the medical electrical System to the
MSO.
•An additional MSO or extension cord shall not be connected to the
medical electrical System.
•MSOs (if provided with the medical electrical System) shall only be
used for supplying power to equipment that is intended to form part
of the medical electrical System.
•Avoid any unnecessary strain on the mains power supply cord.
•When adjusting the height of the display unit on the EchoNous AI
Station, it is important to safely manage the DC power cord to avoid
damage to the cord and risk of electric shock.
•Cord wrap must be installed as the lowest component on the AI
Station in order to protect the handle bar assembly against falling
down into the caster base.

Important Information
12 EchoNous Bladder & Vein User Guide
Cautions
•When opening the collar handles for components on the AI Station
Mobile Stand, it is important to support the component’s weight to
avoid damage or injury from falling components.
•After storage at extreme temperatures, check the transducer surface
temperature before applying to a patient. A cold or hot surface may
burn a patient.
•Avoid musculoskeletal strain with prolonged use of the System.
•Do not incinerate or discard the device in general waste at end of life.
The lithium battery is a potential environmental and fire safety
hazard.
•The EchoNous System complies with the requirements of EN IEC
60601-1 Edition 3.1. To avoid the risk of injury or electrical shock,
comply with all safety instruction and warnings.
•The EchoNous System complies with the Electromagnetic
Compatibility requirements of AS/NZ CISPR 11:2004 and EN IEC
60601-1-2:2014. However, electronic and mobile communications
equipment may transmit electromagnetic energy through air and
there is no guarantee that interference will not occur in a particular
installation or environment. Interference may result in artifacts,
distortion, or degradation of the ultrasound image. If the System is
found to cause or respond to interference, try re-orienting the
System or the affected device, or increasing the separation distance
between the devices. Contact EchoNous customer support or your
EchoNous distributor for further information
•When using the optional mobile stand, the EchoNous System can be
susceptible to ESD and may require manual intervention. If ESD
results in an error, unplug the probe and plug back in to restore
operation.
•The ALARA principle (As Low As Reasonably Achievable) should be
employed for all medical ultrasound exposure.
•Federal (USA) law restricts this device to sale by or on the order
of a physician.
•Ultrasound transducer crystals are fragile and are easily
damaged if knocked, dropped or excessively vibrated.
•Avoid unnecessary bending or winding of the connecting
cable.
•The EchoNous System display batteries should be charged
every six months at a minimum, even if you are not using your
device. When storing for greater than 3 days, store at ambient
or cooler temperature.
•Use only recommended disinfection methods.
•Use abrasive cleaners, isopropyl alcohol or solvents sparingly,
and if used immediately clean and remove residual substances
from the System.

EchoNous Bladder & Vein User Guide 13
Cautions, warnings, and contraindications
Contraindications
•The System is designed for transcutaneous scanning only. Do not attempt intra-cavity imaging; in particular,
trans-esophageal, trans-vaginal and trans-rectal scans are contraindicated.
•The System is not intended for ophthalmic use or any use causing the acoustic beam to pass through the eye.
•Do not heat sterilize any part of the System.
•Minimize application of alcohol based disinfectant to colored
overmold materials. Long term use may result in material
degradation. If alcohol based disinfectant is applied to the
overmold, immediately remove by wiping with a damp cloth.
•Only operate, charge and store the System within the
approved environmental parameters.
•The System contains sensitive components and circuits.
Failure to observe proper static control procedures may result
in damage to the System. Any faults should be reported to
EchoNous customer support or your EchoNous distributor for
repair.

Important Information
14 EchoNous Bladder & Vein User Guide
Labeling
Symbol EchoNous Description
SDO Title, Ref. No.,
Standard
Indicates device
manufacturer
Includes name and address
of the manufacturer
Manufacturer
Ref. No. 5.1.1
ISO 15223-1
Medical devices - Symbols to
be used with medical device
labels, labeling and
information to be supplied -
Part 1: General requirements
Manufacturer's declaration
of product compliance with
applicable EEC directives
and the Notified Body
reference number
CE Marking
Ref. Appendix 12
93/42/EEC EU Medical
Device Directive
UL Classified.
Medical - General medical
equipment as to electrical
shock, fire and mechanical
hazards only
in accordance with ANSI/
AAMI ES 60601-1 (2005) +
AMD (2012)/CAN/CSA-C22.2
No. 6060-1 (2008) + (2014).
E509516
Medical - Refurbished
general medical equipment
as to electrical shock, fire and
mechanical hazards only
in accordance with ANSI/
AAMI ES 60601-1 (2005) +
AMD (2012)/CAN/CSA-C22.2
No. 6060-1 (2008) + (2014).
E509516
None
Tested to comply with FCC
standards
None
This manual suits for next models
1
Table of contents
Other EchoNous Medical Equipment manuals

EchoNous
EchoNous KOSMOS User manual

EchoNous
EchoNous KOSMOS User manual

EchoNous
EchoNous Kosmos Torso-One User manual

EchoNous
EchoNous Kosmos AI Station 2 User manual
EchoNous
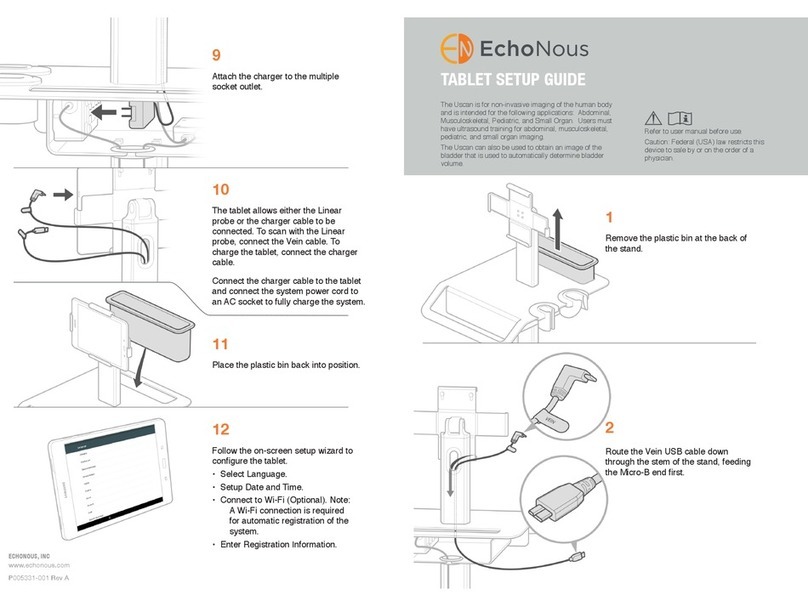
EchoNous Uscan User manual
EchoNous
EchoNous Uscan User manual
EchoNous
EchoNous KOSMOS User manual

EchoNous
EchoNous Kosmos Grab and Go on AI Stand User manual
EchoNous
EchoNous KOSMOS User manual