EDAN INSTRUMENTS SE-2003 Series User manual

I
About this Manual

I
P/N: 01.54.456520
MPN: 01.54.456520019
Release Date: April 2020
© Copyright EDAN INSTRUMENTS, INC. 2014-2020. All rights reserved.
Statement
This manual will help you understand the operation and maintenance of the product better. It is
reminded that the product shall be used strictly complying with this manual. User's operation
failing to comply with this manual may result in malfunction or accident for which Edan
Instruments, Inc. (hereinafter called EDAN) can not be held liable.
EDAN owns the copyrights of this manual. Without prior written consent of EDAN, any
materials contained in this manual shall not be photocopied, reproduced or translated into other
languages.
Materials protected by the copyright law, including but not limited to confidential information
such as technical information and patent information are contained in this manual, the user shall
not disclose such information to any irrelevant third party.
The user shall understand that nothing in this manual grants him, expressly or implicitly, any
right or license to use any of the intellectual properties of EDAN.
EDAN holds the rights to modify, update, and ultimately explain this manual.
Responsibility of the Manufacturer
EDAN only considers itself responsible for any effect on safety, reliability and performance of
the equipment if:
Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
persons authorized by EDAN, and
The electrical installation of the relevant room complies with national standards, and
The instrument is used in accordance with the instructions for use.

II
Terms Used in this Manual
This guide is designed to give key concepts on safety precautions.
WARNING
A WARNING label advises against certain actions or situations that could result in personal
injury or death.
CAUTION
A CAUTION label advises against actions or situations that could damage equipment, produce
inaccurate data, or invalidate a procedure.
NOTE
A NOTE provides useful information regarding a function or a procedure.

III
Table ofContents
Chapter 1 Safety Guidance ...........................................................................................................1
1.1 Indications for Use/Intended Use...........................................................................................1
1.2 Warnings and Cautions..........................................................................................................1
1.2.1 Safety Warnings..............................................................................................................2
1.2.2 Alkaline Battery Care Warnings.....................................................................................3
1.2.3 General Cautions.............................................................................................................3
1.3 List of Symbols......................................................................................................................4
Chapter 2 Introduction..................................................................................................................7
2.1 Appearance.............................................................................................................................8
2.2 Data Storage...........................................................................................................................9
2.3 SD Card Loading and Unloading...........................................................................................9
2.4 Battery Loading....................................................................................................................10
2.5 Features................................................................................................................................11
Chapter 3 Operation Preparations.............................................................................................12
3.1 Requested Materials.............................................................................................................12
3.2 Preparing the Patient............................................................................................................12
3.2.1 Instructing the Patient ...................................................................................................12
3.2.2 Cleaning the Skin..........................................................................................................13
3.3 Connecting the Patient Cable to the Recorder and Electrodes.............................................13
3.4 Attaching the Electrodes to the Patient................................................................................13
3.4.1 Electrode Placement......................................................................................................13
3.4.2 Attaching the Electrodes...............................................................................................16
Chapter 4 ECG Sampling............................................................................................................17
4.1 Overview..............................................................................................................................17
4.2 Start ECG Sampling.............................................................................................................18
4.3 Sampling… ..........................................................................................................................19
4.4 Stop Sampling......................................................................................................................19
4.5 Data Transmission................................................................................................................21
Chapter 5 System Setting ............................................................................................................22
5.1 Basic Setting ........................................................................................................................22
5.2 Advanced Setting.................................................................................................................23
Chapter 6 Hint Information........................................................................................................24
Chapter 7 Cleaning, Care and Maintenance.............................................................................25
7.1 General Points......................................................................................................................25
7.2 Cleaning ...............................................................................................................................25

IV
7.2.1 Cleaning the Recorder...................................................................................................26
7.2.2 Cleaning the Patient Cable............................................................................................26
7.3 Disinfection..........................................................................................................................26
7.3.1 Disinfecting the Recorder .............................................................................................27
7.3.2 Disinfecting the Patient Cable.......................................................................................27
7.4 Care and Maintenance..........................................................................................................27
7.4.1 Visual Inspection...........................................................................................................27
7.4.2 Maintenance of the Recorder and the Patient Cable.....................................................29
Chapter 8 Accessories..................................................................................................................31
Chapter 9 Warranty and Service ...............................................................................................32
9.1 Warranty...............................................................................................................................32
9.2 Contact Information.............................................................................................................32
Appendix 1 Technical Specifications..........................................................................................33
A1.1 Safety Specifications.........................................................................................................33
A1.2 Environment Specifications ..............................................................................................34
A1.3 Physical Specifications......................................................................................................34
A1.4 Battery Specifications .......................................................................................................34
A1.5 Performance Specifications...............................................................................................34
Appendix 2 EMC Information....................................................................................................36
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 1 -
Chapter 1 Safety Guidance
This chapter provides important safety information related to the use of SE-2003/SE-2012 series
Holter System Recorder.
1.1 Indications for Use/Intended Use
The SE-2003&SE-2012 Series Holter System (including recorder and analysis software) is
intended to record, analyze, display, edit and generate report of ambulatory ECG. The Holter
System is intended to be used by trained personnel under the direction of doctors. The analysis
results are offered to doctors on an advisory basis only. The Holter System is intended for adult,
pediatric patients including infants weighing less than 10 kg.
It can be used for the following indications:
1. Evaluation of symptoms suggesting arrhythmia or myocardial ischemia.
2. Evaluation of patients for ST segment changes.
3. Evaluation of drug response in patients taking anti-arrhythmic medications.
4. Evaluation of patients with pacemakers.
WARNING
1. This recorder is not designed for internal use or direct cardiac application.
2. This recorder is not intended for treatment.
3. The results given by the recorder should be examined based on the overall clinical
condition of the patient, and they can not substitute for regular checking.
1.2 Warnings and Cautions
Consideration of Safety and Efficiency
The dependability of the recorder depends on the proper operation in accordance with
operation and maintenance guidance given in the manual.
The lifetime of the recorder mainly depends on the components validity, which is 5 years
under normal conditions. If components validity exceeds the time limit, the possibility of
aging failure will increase and it may lead to operational failure.
The measure result provided by the recorder is just a reference for physician. The final
diagnosis is made by physician.
The Holter recorder belongs to type CF equipment , and defibrillation shouldn't be
performed on a patient wearing the recorder.

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 2 -
In order to use the recorder safely and effectively, and avoid possible dangers caused by improper
operation, please read through the user manual and be sure to be familiar with all functions of the
equipment and proper operation procedures before use.
Please pay more attention to the following warning and caution information.
1.2.1 Safety Warnings
WARNING
1. The recorder is intended to be used by qualified physicians or personnel
professionally trained. They should be familiar with the contents of this user manual
before operation.
2. Only qualified service engineers can install this recorder, and only service engineers
authorized by the manufacturer can open the shell. Otherwise, safety hazards may
happen.
3. EXPLOSION HAZARD - Do not use the recorder in the presence of flammable
anesthetic mixtures with oxygen or other flammable agents.
4. Do not use this recorder in the presence of high static electricity or high voltage
equipment which may generate sparks.
5. Prevent any liquid from seeping into the recorder; otherwise the safety and the
performance of the recorder can not be guaranteed.
6. Only the patient cable and other accessories supplied by the manufacturer can be
used. Or else, the performance and electric shock protection can not be guaranteed.
7. Make sure that all electrodes are connected to the patient correctly before operation.
8. Wrap and secure excess cabling to reduce the risk of entanglement or strangulation.
9. Ensure that the conductive parts of electrodes and associated connectors, including
neutral electrodes, do not come in contact with earth or any other conducting objects.
10.The disposable electrodes can only be used for one time.
11.The use of patient cable and other accessories not supplied by the manufacturer may
result in increased emissions or decreased immunity of the equipment.
12.The use of equipment that applies high frequency voltages to the patient (including
electrosurgical equipment and some respiration transducers) is not supported and
may produce undesired results. Disconnect the patient data cable from the recorder,
or detach the leads from the patient prior to performing any procedure that uses high
frequency surgical equipment.
13.If multiple instruments are connected to a patient, the sum of the leakage currents
may exceed the limits given in the IEC/EN 60601-1 and may pose a safety hazard.
Consult your service personnel.

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 3 -
WARNING
14.The belt, Lanyard, and protective case cannot contact the skin directly.
15.The recorder shall not be serviced or maintained while in use with a patient.
16.The medical electrical equipment needs to be installed and put into service according
to Appendix 2 EMC information.
17.The equipment should not be used adjacent to or stacked with other equipment, refer
to the recommended separation distances provided in Appendix 2 EMC Information.
18.Portable and mobile RF communications equipment can affect medical electrical
equipment, refer to the recommended separation distances provided in Appendix 2
EMC Information.
1.2.2 Alkaline Battery Care Warnings
WARNING
1. Do not heat or splash the battery or throw it into fire or water.
2. Do not destroy the battery; Do not pierce battery with a sharp object such as a needle.
Do not hit with a hammer, step on or throw or drop to cause strong shock. Do not
disassemble or modify the battery.
3. When leakage or foul smell is found, stop using the battery immediately. If your skin or
cloth comes into contact with the leakage liquid, cleanse it with clean water at once. If
the leakage liquid splashes into your eyes, do not wipe them. Irrigate them with clean
water first and go to see a doctor immediately.
4. Properly dispose of or recycle the depleted battery according to local regulations.
5. Remove the battery from the recorder when the recorder isn't used for a long time.
1.2.3 General Cautions
CAUTION
1. Federal (U.S.) law restricts this device to sale by or on the order of a physician.
2. Avoid liquid splash and excessive temperature. The temperature must be kept
between 5 ºC and 45 ºC during operation, and it should be kept between -20 ºC and
55 ºC during transportation and storage.
3. The time required for the Holter recorder between uses to warm from the minimum
storage temperature till it is ready for intended use is at least 2 hours and that
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 4 -
required to cool from the maximum storage temperature till it is ready for intended use
is at least 2 hours.
4. Do not use the recorder in a dusty environment with bad ventilation or in the presence
of corrosive.
5. Make sure that there is no intense electromagnetic interference source around the
equipment, such as radio transmitters or mobile phones etc. Attention: large medical
electrical equipment such as electrosurgical equipment, radiological equipment and
magnetic resonance imaging equipment etc. is likely to bring electromagnetic
interference.
6. The recorder and accessories are to be disposed of according to local regulations
after their useful lives. Alternatively, they can be returned to the dealer or the
manufacturer for recycling or proper disposal. Batteries are hazardous waste. Do
NOT dispose of them together with house-hold garbage.At the end of their lives hand
the batteries over to the applicable collection points for the recycling of waste
batteries. For more detailed information about recycling of this product or battery,
please contact your local Civic Office, or the shop where you purchased the product.
7. Prevent babies or children from swallowing small components, e.g. the battery.
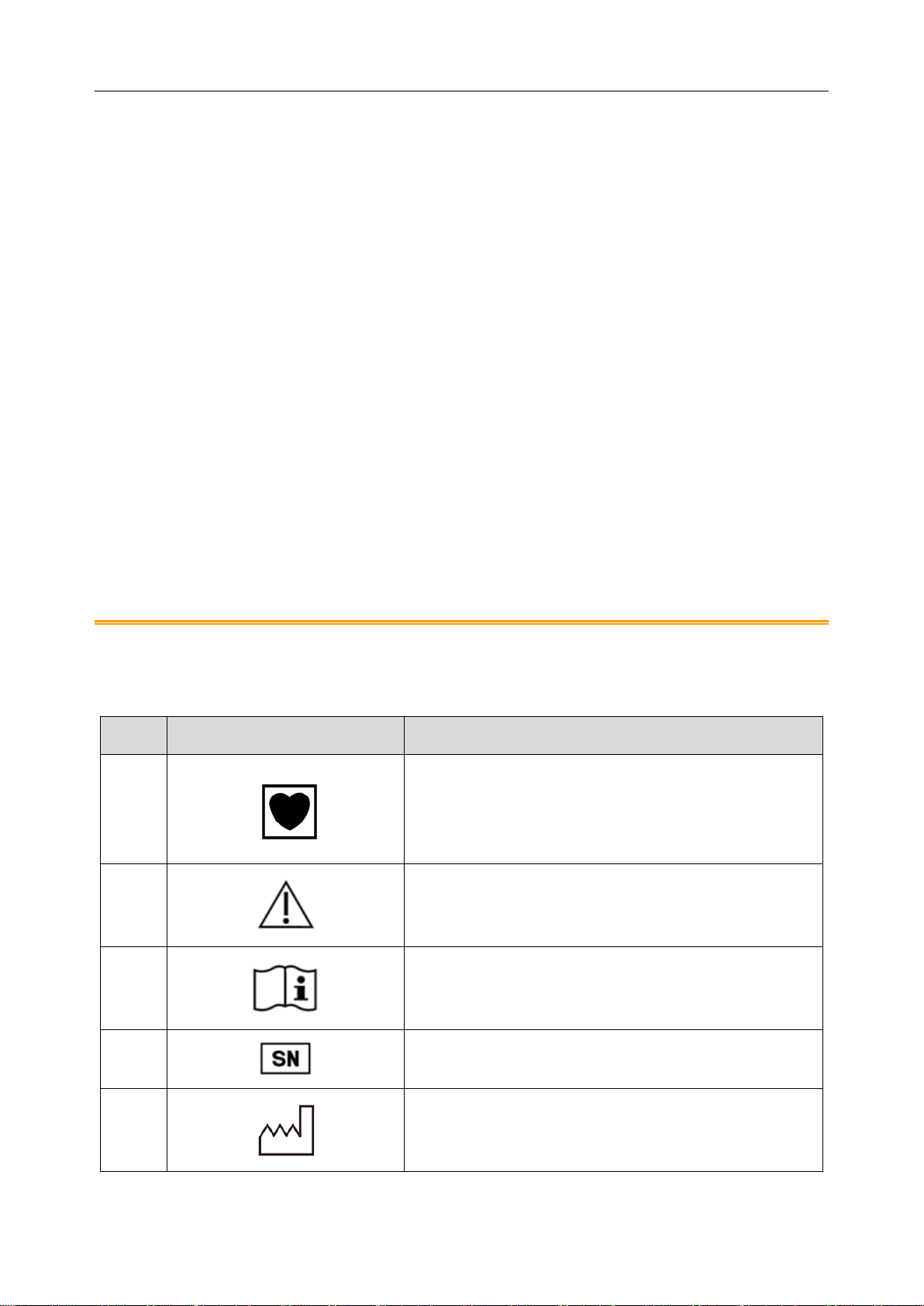
1.3 List of Symbols
NO.
Symbol
Description
1
TYPE CF APPLIED PART
2
Caution
3
Consult operating instructions
4
SERIAL NUMBER
5
Date of manufacture
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 5 -
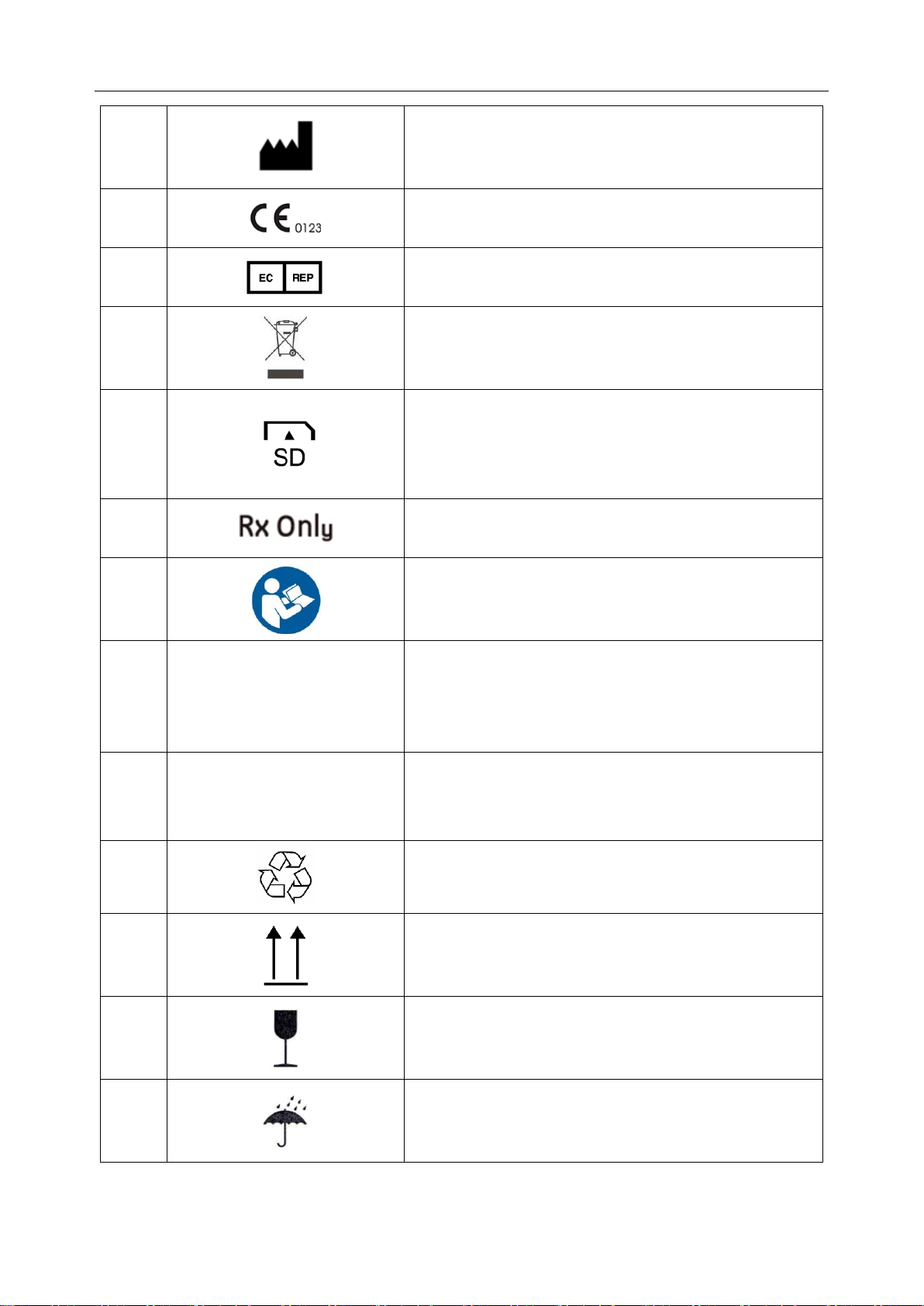
6
MANUFACTURER
7
CE marking
8
AUTHORISED REPRESENTATIVE IN THE
EUROPEAN COMMUNITY
9
Disposal method
10
Insert SD card in the direction the arrow indicates.
11
Caution: Federal (U.S.) law restricts this device to sale
by or on the order of a physician.
12
Refer to instruction manual/booklet
(Background: Blue; Symbol: White)
13
IP 27
Protected against solid foreign objects of 12.5 mm Ø
and greater
Protected against the effects of temporary immersion in
water
14
IP 22
Protected against solid foreign objects of 12.5 mm Ø
and greater
Protected against dripping water when tilted up to 15o
15
General symbol for recovery/recyclable
16
This way up
17
Fragile, handle with care
18
Keep away from rain

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 6 -
19
Stacking limit by number
20
Handle with care
21
Do not step on
22
Front
NOTE: The user manual is printed in black and white.

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 7 -
Chapter 2 Introduction
The manual mainly describes how to operate and maintain SE-2003/SE-2012 series Holter
System Recorder (thereafter referred as SE-2003/SE-2012 series). The SE-2003/SE-2012 series
Holter Analysis System consists of the recorder and analysis software. For the operation of
analysis software, refer to software manual.
The SE-2003/SE-2012 series have four recorder models: SE-2003, SE-2012, SE-2003A, and
SE-2012A. The difference lies in the SD card. SE-2003 and SE-2012 recorders can be inserted
with micro-SD card while SE-2003A and SE-2012A inserted with SD card.
Ambulatory Electrocardiogram (Holter) detection technology is an effective tool to detect
cardiovascular without the inference of distance, time, environment, the restriction of body
position and activity. It can detect large amount of ECG information and is unique in capture of
transient myocardial ischemia and diagnosis of transient arrhythmia.
SE-2003/SE-2012 series is powered by an AAA battery. SD card is used as storage media and
LCD screen is used to set parameters and check wave quality. With a normal AAA alkaline
battery, SE-2012/SE-2012A can continuously record non-compressed and full-disclosure 12-lead
ECG data for 24~48 hours or 3-channel ECG data for 24~96 hours . With a normal AAA
alkaline battery, SE-2003/SE-2003A can continuously record non-compressed and full-disclosure
3-channel ECG data for 24~96 hours. With a Li-Fe battery, SE-2003/SE-2003A can continuously
record non-compressed and full-disclosure 3-channel ECG data for 7 days. SE-2003/SE-2012
series can detect and record pacemaker pulse information.
The Holter System is intended to be used in hospitals and clinics by trained personnel under the
direction of doctors. The patient wears the Holter Recorder with the help of the doctor and the
recorder records ECG data for at least 24 hours continuously. The patient may return home
during the recording process. Finally, the doctor analyzes the ECG data recorded and makes
diagnosis.
NOTE:
1. Long-time recording analysis should be supported by software with corresponding
functions.
2. The manufacturer recommends the type of LiFe battery that can be used.
Recommended LiFe battery: ENERGIZER L92
3. The manufacturer does not provide Ni-MH rechargeable battery and its charger.

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 8 -
2.1 Appearance
Please find appearance of SE-2003/SE-2012 Holter System Recorder below:
When powered on, the main screen displays the time, battery capacity, and basic device
information.
The confirmation key serves as patient events key in monitoring process. If patients feel
uncomfortable or want to record time of symbolic meaning (start to do exercise, begin to sleep,
etc.), press the key and the recorder will record the time.
NOTE:
1. Pay attention to the plug direction. Please insert the side with marker toward recorder
with appropriate force.
2. Press Confirmation key to restart SE-2003/SE-2012 series if it shuts off automatically.
There is no need to load battery again.
CAUTION
Do not sway plug during use. Plug may fall off and that will cause record failure and even
damages to socket.
Confirmation Key
Long press to power on
Confirm operation
Record an event
Change cursor status
Multifunctional
Socket
LCD Screen
Direction Keys
Move the cursor

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 9 -
2.2 Data Storage
SE-2003/SE-2012 series stores ECG data in Secure Digital card (SD card) which will be analyzed
by Holter analysis software after finishing recording.
Only the SD card specified by manufacturer can be used in SE-2003/SE-2012 series. If you need
to add or replace SD card, please contact manufacturer or dealer. Do not insert incompatible or
unknown SD card into SE-2003/SE-2012 series recorder. That is to avoid unnecessary damages.
Capacity of SD card accompanied with recorder is 1G. If the SD card is not provided together
with recorder, please contact the manufacturer or distributor.
CAUTION
SD card is a light and precise device. Do not knock or bend it or insert articles into the
jacks. Keep the SD card in the recorder and it helps to prevent foreign matters from
falling into SD card slot.
2.3 SD Card Loading and Unloading
SD card holder is push-push structure.
Load:
Face front of SD card with a cut angle toward back cover of recorder, slightly push it into SD
card slot until the end of SD card and card slot are at the same level. Release the finger and let SD
card automatically draw back 1 mm or so. It means SD card is in the right place.
Unload:
Use finger to push SD card inside until its end and card slot are at the same level. Release the
finger and SD card will automatically eject 5 mm or so. Use fingernail to catch the end of SD
card and slightly pull it out.
CAUTION
1. Do not load SD card with too much force. If you feel resistance, please check load
direction or check if any articles block in the slot.
2. The first step to unload SD card is to push card inside and then let it pop up
automatically. Never pull out the card by force before it pops up. It may cause
damage to recorder and SD card.
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 10 -
2.4 Battery Loading
SE-2003/SE-2012 series is powered by one AAA battery. Large capacity AAA alkaline battery is
recommended. Press mark on battery compartment on the back of recorder by thumb and
push outwards with force. The cover of battery compartment will open. Load one AAA battery
according to polarity indication inside battery compartment.
NOTE: There is battery type setting in the setting menu of SE-2003/SE-2012 series:
alkaline battery and Ni-MH battery. The purpose is to give more exact warning
message on battery under-voltage according to discharge performance of
different battery types. Please set the menu according to used battery type.
WARNING
1. Start a new patient with a new battery, to delete all old data and to reset the device's
settings to fit the new patient.
2. Take out battery if the recorder is not to be used for more than an hour. Or else
damage from corrosion could result.
3. Do not throw off used or scrapped batteries. Observe operation instructions and local
laws for battery disposal.

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 11 -
2.5 Features
SE-2012 Holter System Recorder is able to create standard 12 leads or 3 channels full
ECG data;
EDAN's unique multi-channel pacemaker detect circuit effectively prevents wrong
detection of pacemaker signal caused by all kinds of artifacts (such as movement,
polarized voltage and skin impedance) and missing detection caused by software
detecting only. The detection sensitivity can reach 10-4 second.
1.92" full color screen plus a 3-key keyboard make it easy to set recording parameters of
the Holter System Recorder. Real time ECG waves display helps to check electrode
placement quality. During recording, you can switch to ECG display window at any time
to master ECG recording situation;
Multi-languages menu, easy and friendly to operate;
Real-time clock, real-time display of year, month and date; the recording time is actual
time, which prevents trouble and poor accuracy caused by recording manually.
E-label: Support either registration in analysis software or entry of patient ID by recorder
keyboard. Basic information (patient ID, name, gender, age) of patients is written into
data package before making records. In this way recorders used by different patients will
not be mixed up when reviewing data package. Numerous data are included in the
package like hospital, signal channel, sampling rate, event information, recording date
and time, so as to facilitate data management and exchange.
Leads-off warning: Poor electrode connection will be warned.
Power supply management, prompt of detection of battery under voltage; the power
supply will shut off automatically after long time idling (15 minutes after last keyboard
response) or 30 minutes after end of recording so as to save battery capacity and avoid
battery leakage.
Flexible communication mode, support plug-and-play SD card as well as USB 2.0
high-speed direct communication. SD card helps to speed up patient turnaround and is
convenient to maintain so as to alleviate users' burden; on the other hand, USB 2.0
high-speed communication mode is simple and easy.
Events button precisely records event time.

SE-2003&SE-2012 Series Holter System Recorder User Manual
- 12 -
Chapter 3 Operation Preparations
WARNING
Before use, the recorder, patient cable and electrodes should be checked. Replace them
if there is any evident defectiveness or aging which may impair the safety or the
performance, and make sure that the equipment is in proper working condition.
3.1 Requested Materials
1. Recorder, patient cables, leads, SD card;
2. An analysis system able to make electronic labeling registration or other specialized
software;
3. 10 (or 5, 7) disposable electrodes;
4. 1 AAA Alkaline battery or 1 fully charged Ni-MH rechargeable battery;
5. Patient log, pen;
6. Other supplementary materials like alcohol, medical adhesive plaster.
3.2 Preparing the Patient
3.2.1 Instructing the Patient
Give necessary guidance to patients before they leave:
1. Show them how to use events button and explain when to press the button;
2. Explain to the patients or nursing staff the importance of registering patient log timely and
completely. Introduce writing format, contents, recording time, location, activities and
self-feeling in detail;
3. Warn patients:
Do not immerse the recorder in water for more than 30 minutes or to depth more than 1 m.
Do not touch electrodes or unplug leads.
Do not open recorder. Do not take out battery or SD card;
Do not place mobile phones, TV or other electrical appliances one meter around the
recorder.
Do not place heat source like heater close to the recorder.
4. Tell them how to record an event when they feel extremely uncomfortable;
5. Let them revisit 24 hours (48 hours or longer) later.
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 13 -
3.2.2 Cleaning the Skin
Thorough skin preparation is very important. The skin is a poor conductor of electricity and
frequently creates artifacts that distort the ECG signals. By performing methodical skin
preparation, you can greatly reduce the possibility of noise caused by muscle tremor and baseline
drift, ensuring high-quality ECG waves. There is natural resistance on the skin surface due to dry,
dead epidermal cells, oils and dirt.
To Clean the Skin
1. Shave hair from electrode sites, if necessary. Excessive hair prevents a good connection.
2. Wash the area thoroughly with soap and water.
3. Dry the skin with a gauze pad to increase capillary blood flow to the tissues and to remove
the dead, dry skin cells and oils.
NOTE: If you don't have enough time for the steps above, you can use gauze to scrub the
electrode sites to remove the dead, dry skin cells and oils and increase capillary
blood flow to the tissues.
3.3 Connecting the Patient Cable to the Recorder and Electrodes
WARNING
The performance and electric shock protection can be guaranteed only if the original
patient cable and electrodes of the manufacturer are used.
Follow the arrow on the patient cable to connect it to the cable socket on the top of recorder.
Make sure that the connection is tight.
3.4 Attaching the Electrodes to the Patient
3.4.1 Electrode Placement
WARNING
1. Make sure that all electrodes are connected to the patient correctly before operation.
2. Make sure that the conductive parts of electrodes and associated connectors,
including neutral electrode, do not come in contact with earth or any other conducting
objects.
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 14 -
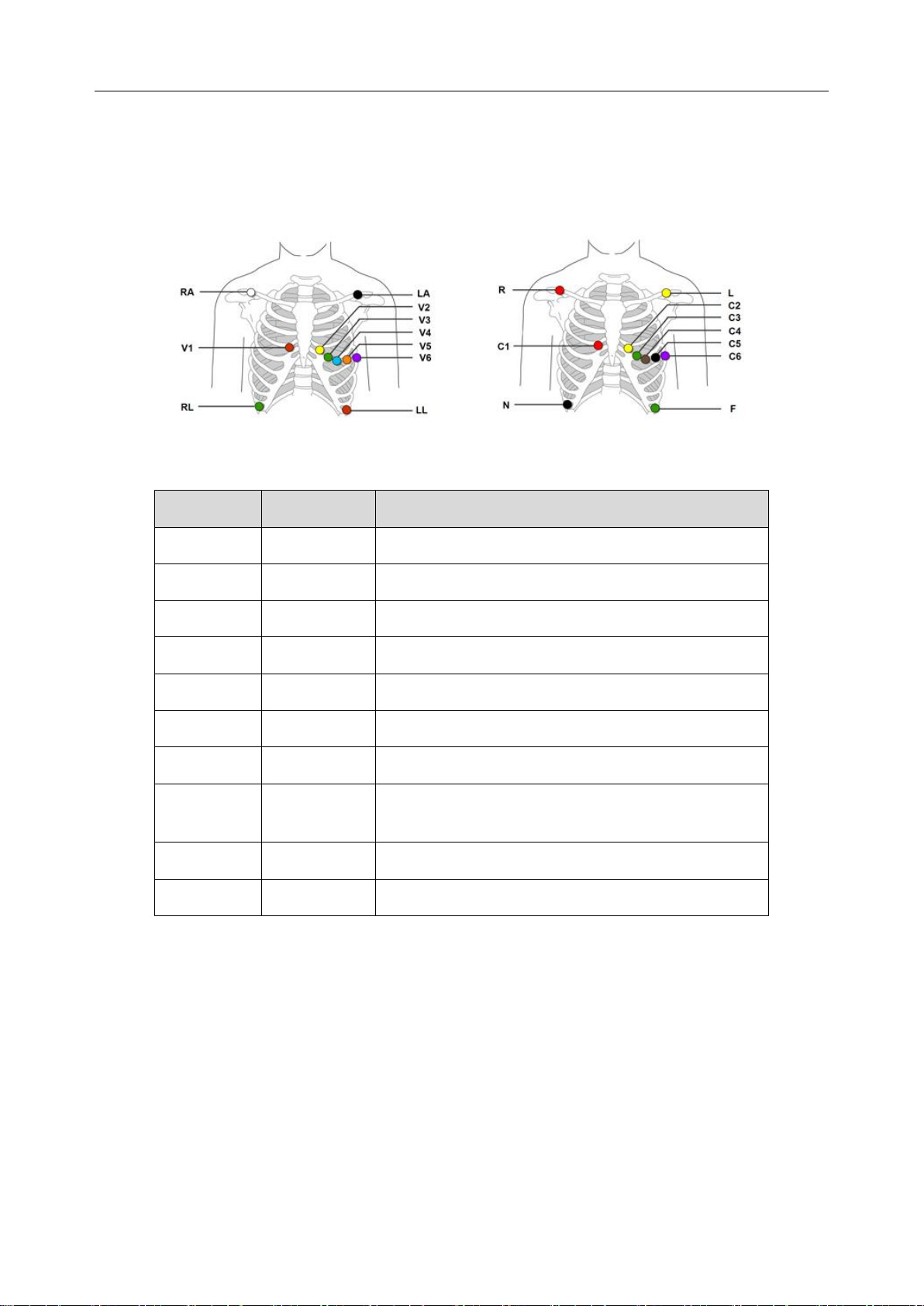
3.4.1.1 10 Electrodes 12 Leads
10-electrode standard lead wires are utilized by the recorder (12-channel) to create a 12-lead ECG
signal.
AHA IEC
IEC
AHA
Electrode Placement
Red R
White RA
Below clavicle, close to right shoulder.
Yellow L
Black LA
Below clavicle, close to left shoulder.
Black N
Green RL
Lower right rib margin over bone.
Green F
Red LL
Lower left rib margin over bone.
Red C1
Red V1
Right of Mid-Clavicular line 4th rib
Yellow C2
Yellow V2
Left of Mid-Clavicular line 4th rib
Green C3
Green V3
In the middle of V2 and V4.
Brown C4
Blue V4
Brown V4: Fifth intercostal space on the left
mid-clavicular line
Black C5
Orange V5
Left Anterior Axillary line, the same level of V4
Purple C6
Purple V6
Left Middle Axillary line, the same level of V4
SE-2003&SE-2012 Series Holter System Recorder User Manual
- 15 -
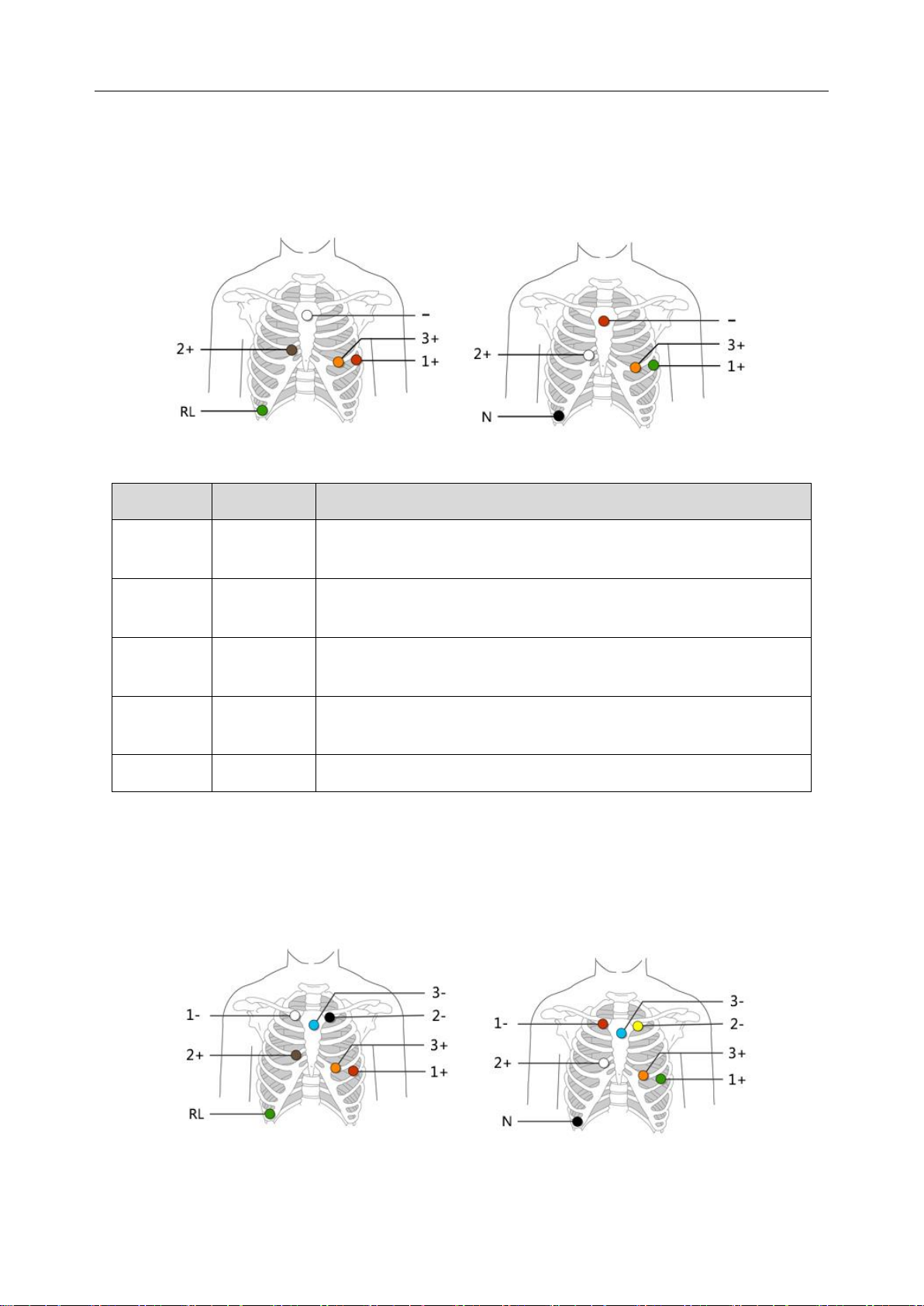
3.4.1.2 5 Electrodes 3 Channels
Five-electrode-three-channel lead wires are utilized by the recorder to create a 3-channel ECG
signal.
AHA IEC
IEC
AHA
Electrode Placement
Red
(COM-)
White
(COM-)
Right Manubrial border of the Sternum.
Green
(CH1+)
Red
(CH1+)
Fifth intercostal space on the left anterior axillary line, equivalent to
V5 breast lead.
White
(CH2+)
Brown
(CH2+)
Right of Xiphoid Process on the rib, equivalent to V1.
Orange
(CH3+)
Orange
(CH3+)
Left fifth rib, equivalent to V3.
Black(N)
Green (RL)
Lower right rib margin over bone.
3.4.1.3 7 Electrodes 3 Channels
Seven-electrode-three-channel lead wires are utilized by the recorder to create a 3-channel ECG
signal.
AHA IEC
This manual suits for next models
5
Table of contents
Other EDAN INSTRUMENTS Medical Equipment manuals

EDAN INSTRUMENTS
EDAN INSTRUMENTS SE-3 User manual

EDAN INSTRUMENTS
EDAN INSTRUMENTS VE-100 User manual

EDAN INSTRUMENTS
EDAN INSTRUMENTS VE-1010 User manual

EDAN INSTRUMENTS
EDAN INSTRUMENTS SE-3 User manual

EDAN INSTRUMENTS
EDAN INSTRUMENTS MT-206 User manual
EDAN INSTRUMENTS
EDAN INSTRUMENTS CADENCE User manual

EDAN INSTRUMENTS
EDAN INSTRUMENTS iM8s User manual

EDAN INSTRUMENTS
EDAN INSTRUMENTS SE-12 Express Quick start guide