USER GUIDE
V.1.0.R7
3. INDICATIONS
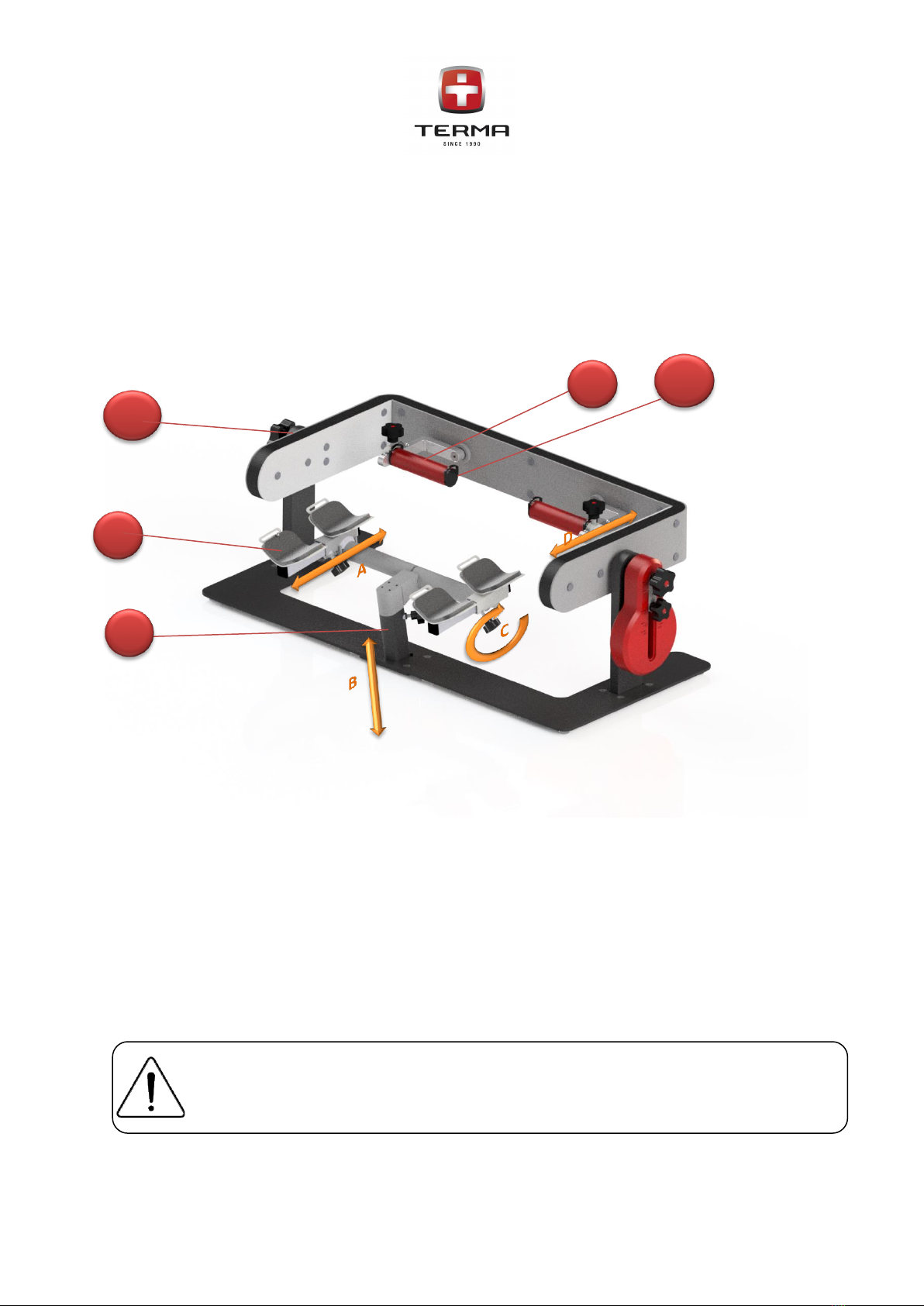
PICTOR device is dedicated for adults. It is dedicated for kinesiotherapy post traumatic injuries.
•Injuries to ligaments and cartilage,
•Muscle and ligament strains,
•Twists of the wrist joint,
•Dislocations in the area of wrist joint,
•Breaks of the wrist joint,
•Neurological problems causing upper limb disfunctions.
The device is dedicated to be used in rehabilitation facilities and at home on the basis of a loan from the facility.
The condition for safe and effective use is the constant supervision of a physiotherapist over the course of
exercises, ranges of motion and the applied resistance.
4. PROPONOWANE FORMY REALIZACJI TERAPII
Urządzenie jest dostarczane do klienta w formie nie wymagających żadnych czynności przystosowawczych
urządzenie do rozpoczęcia eksploatacji.
Umożliwia realizację następujących ćwiczeń:
Flexion and extension of the wrist