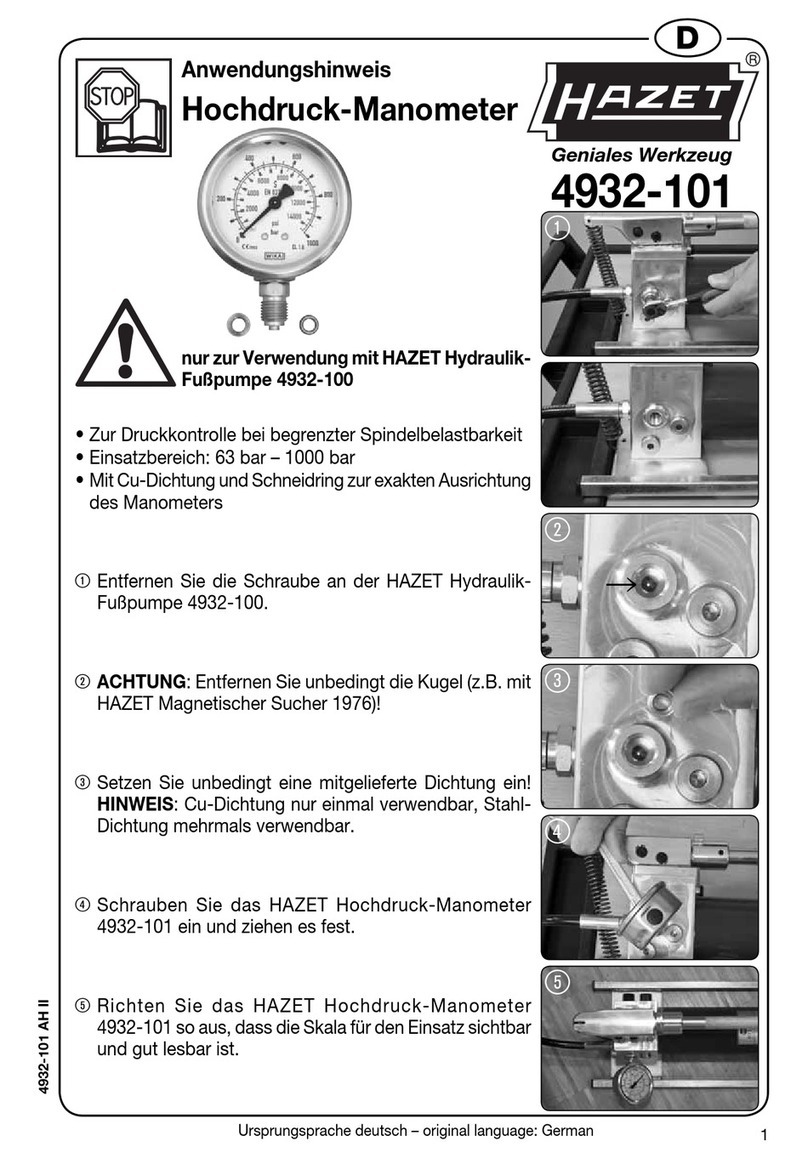
EIJKELKAMP 14.36 User manual

1
OXYGEN DIFFUSION METER
All it takes for environmental research
P.O. Box 4, 6987 ZG Giesbeek,
the Netherlands
T+31 313 88 02 00
F+31 313 88 02 99
I www.eijkelkamp.com
M1.14.36.E
© December 2008
OPERATING INSTRUCTIONS
Contents
On these operating instructions ................................................................................................................................2
Introduction ............................................................................................................................................................2
1 Description ..........................................................................................................................................................2
1.1 Oxygen diffusion rate ................................................................................................................................2
1.2 ODR meter ................................................................................................................................................3
1.3 Pt-electrode...............................................................................................................................................3
1.3.1 Preparation...........................................................................................................................................3
1.3.2 Maintenance ........................................................................................................................................4
1.4 Reference electrode ...................................................................................................................................4
1.4.1 Commercial electrodes .........................................................................................................................4
1.4.2 Preparation for use...............................................................................................................................4
1.4.3 Maintenance ........................................................................................................................................4
1.4.4 Storage ................................................................................................................................................4
1.4.5 Preparation of a used electrode ............................................................................................................5
1.4.5.1 Before (re-)generating, the electrode must be cleaned ..................................................................5
1.4.5.2 Generating an Ag-AgCl electrode.................................................................................................5
1.5 Brass electrode ..........................................................................................................................................5
1.5.1 Maintenance ........................................................................................................................................5
2 Measurements .....................................................................................................................................................6
2.1 Measurement of Redox..............................................................................................................................6
2.2 Measurement of ODR................................................................................................................................6
2.3 Checking the instrument ...........................................................................................................................7
3 Storage, transport................................................................................................................................................8
4 Specications.......................................................................................................................................................8
Literature ............................................................................................................................................................9
Figures ..........................................................................................................................................................10

2
On these operating instructions
If the text follows a mark (as shown on the left), this means that an important instruction fol-
lows.
If the text follows a mark (as shown on the left), this means that an important warning fol-
lows relating to danger to the user or damage to the apparatus.The user is always responsible
for its own personal protection.
Italic indicated text indicates that the text concerned appears in writing on the display(or must
be typed).
Introduction
Theoxygendiffusionrate(ODR)metermeasuresthemobilityofoxygeninthesoil.Thismobilityisofsignicance
to the availability of oxygen to the plant roots. The method involves the measurement of the electric current nee-
ded for the reduction of all available oxygen at the surface of a cylindrical Pt-electrode in the soil. Thus the oxygen
uxthroughtheair-lledporesandthewaterlmontheelectrodetoazeroconcentrationattheelectrodesurface
is measured.
The transport of oxygen is much faster in air than in water. In a given type of soil the diffusion rate will therefore
depend strongly on the air to water ratio in the pores of the soil-structure. Plant roots require for their growth a
sufcientafuxofoxygenaswillbeprovidedbymoderatelyorwellaeratedsoils.Careshouldbetakentoavoid
any change of aeration at the point of measurement. The ODR-sensor must not cause any compression of the soil.
The soil is a very complex system. The results of an ODR measurement in a soil are highly sensitive to the voltage
between the Pt-electrode and the soil. For comparable results the conditions of measurement should be well de-
nedandcloselycontrolled.TheODRmeterhasbeendesignedtoprovideastabilisedvoltageof0.65Vbetween
thePt-electrodeandtheAg-AgCIreferenceelectrode,and,hence,of0.428VbetweenthePt-electrodeandthe
soil (at 25 °C).
There may be some effect of pH on the outcome of an ODR measurement, but this is usually small for pH ≥3.5.
The design of the ODR meter comprises a voltmeter with a high input impedance. To further exploit this facility the
selector switch has been provided with a special position for the measurement of the Redox potential of the soil.
For an ODR measurement, the platinum electrode should be completely covered by a water
lm. In practice this means that readings should only be taken if the moisture content of the
soil is at eld capacity. In drier soils a Redox measurement offers a good alternative to char-
acterise the soil, because the Redox potential is measured in a high impedance circuit, with a
very low electrical current anyway. The user should be aware that soil is a heterogeneous envi-
ronment, in which measurements will vary. Only average values of several, upto ten or more,
measurements have meaning.
1 Description
1.1 Oxygen diffusion rate
Theinstrumentmeasurestheelectriccurrentneededforaquantitativereductionoftheoxygenowingfromthe
airspaces in the soil to the platinum electrode. The chemical reaction is given by:
O2+ 2H2O + 4e-4 OH-.
!
Text

3
The reduction of one mole of oxygen thus requires four mole electrons which is equivalent to a charge of 4 x
96500 = 386000 Coulombs.
If ODR is the reduction rate of oxygen expressed in grams per second per square meter, this can be done as fol-
lows:
i x 32
ODR = —————
386000 * A
In which i is the current readout in micro-amperes and A the surface area of the electrode in square meters.
In the equation i should be used as a dimensionless value.
Given de surface of the electrode is about 2.37*10-5 m2(or 0.0000237 m2) this results in:
ODR = i * 3.5 micrograms/m2/S
in which i is the dimensionless value of the instrument readout.
So if you read the value 40 in the display ODR becomes 40 * 3.5 = 140 micrograms/m2/s
1.2 ODR meter
The measurement system consists of a carrying case containing an indicating instrument (read-out unit), a plastic
bottle with an Ag-AgCl reference electrode with a KCI salt bridge and a brass counter electrode. The Pt-electrode
and a Riverside auger are delivered separately.
Inthiscongurationthetwofunctionsofthecounterelectrodeareseparated:thereferenceelectrodeisusedto
measure and monitor the potential difference between the Pt-electrode and the soil, and the brass electrode is
usedtoclosetheelectriccircuitforthecurrentneededtoreducetheoxygen.Thebasiccircuitisshowningure1
on page 8.
The electro-chemical processes in the soil depend largely on the applied voltage. At a voltage of about 0.5 volt
between the Pt-electrode and the soil the reduction of oxygen is the dominant process. The magnitude of the cur-
rentwillvarysignicantlywiththevoltage.Itisthereforeimportanttospecifytheappliedvoltageandtokeepthe
voltage within narrow tolerances during measurements.
The potential of the Pt-electrode is measured with respect to an Ag-AgCI reference-electrode that is in electrical
contact with the soil over a salt bridge. This measured value is used to control a voltage regulator circuit that stabi-
lisesthevoltagebetweenthePt-electrodeandthereferenceelectrodeatavalueof0.65V.
As the anodic counter-electrode a brass electrode is used, also placed in direct contact with the soil.
1.3 Pt-electrode
The Pt-electrode is a 6 mm length of Pt wire, hardened with a 10 % addition of Ir. The wire has a diameter of 1,2
mm. The wire is mounted at the tapered end of a 750 mm long rod that facilitates the placement of the electrode
at various depths in the soil. At the top of the rod is a female BNC connector for the connection of the cable to
the read-out unit. A BNC cap is provided to protect the connector from soil, dirt and water when not in use. The
electrodeisoutlinedingure2onpage11.
1.3.1 Preparation
Take the electrode assembly and connect a signal cable to the BNC connector. Take the cover tube from the tip.
Rubtheplatinumtipwiththeglassbrepenntoremoveanyoxide(invisiblebutverydisturbingonredoxmeasu-
rements). For accurate redox measurements follow the instructions of manual M2.18.21.27.E and/or ISO standard
11271.

4
Drill a hole to the desired depth minus 10 mm and insert the electrode-rod into the hole. Exert a slight pressure on
the top of the assembly to bring the electrode to its correct position. Take care not to damage the electrode.
It is important that the electrode is positioned in undisturbed ground.
1.3.2 Maintenance
Wipe the electrode clean with a soft tissue after use and replace the cover tube. In the rare case of very persistent
stains,theuseofthebrepenwillsufce.
ODR electrodes should not be left in the soil for an extended period of time, since a decrease
of readings may occur. It is best to take the electrodes out of the soil after the completion of
one set of measurements and to reinsert them for the next one.
1.4 Reference electrode
Asilver-silverchlorideelectrodelledwithasaturatedKCIsolutionisusedasthereferenceforvoltagemeasure-
ment.TheAg-AgClelectrodesystemismountedontopofaporous,ceramiccupthatislledwithasaturatedKCI
solution to serve as a salt bridge to the soil. The connection between the electrode system and the salt bridge is a
wetjunction.AminuteowofKCIsolutionfromtheelectrodevesselintothesaltbridgeandfromthesaltbridge
into the soil should always be maintained during measurements. The pressure release screw near the top of the
electrode assembly should be left open whenever the electrode is in use. Only when not in use the pressure release
screw may be closed. For the electrical connection a female BNC connector is provided at the top of the electrode
assembly. The electrode vessel is mounted in a screw cap that can be screwed onto a plastic bottle with a KCI solu-
tion. A spare cap is provided to close the bottle when the electrode is in use. The reference electrode and the salt
bridgearedepictedingure3onpage11.
1.4.1 Commercial electrodes
Insteadofthereferenceelectrodesuppliedwiththeinstrument,acommercialAg-AgCIelectrodettedwitha
salt bridge may be used. Though the procedure will not differ much from the one given above, always follow the
instructions of the manufacturer.
1.4.2 Preparation for use
Take the electrode assembly from the plastic bottle and close the bottle with the spare cap. Unscrew the pressure
release screw one turn. Pull off the head of the silver electrode. Fill the vessel with a saturated solution of KCI to a
level 15 mm below the brim. Check that the O-ring in the head is in good shape and replace the head.
1.4.3 Maintenance
RelltheelectrodevesselwithasaturatedKCIsolutionwhenthelevelhasdroppedabout30mmbelowtheinitial
level or if any signs of discolouration (poisoning) of the liquid is noticed. Keep the pressure release screw closed
when the electrode is not in use to prevent unnecessary leakage of KCI. Brush the ceramic of the reference elec-
trode clean between and after use. Use a tooth brush and rinse ample water while doing this.
1.4.4 Storage
Always store the electrode/salt bridge assembly in its plastic bottle with KCI solution. See to it
that the ceramic cup stays free from the bottom. The KCl solution in the storage bottle should
be kept absolutely clean!

5
1.4.5 Preparation of a used electrode
The standard reference electrodes are supplied with a AgCl coating by Eijkelkamp. When the AgCl-coating is visu-
ally damaged or weared after use it must be renewed. The “old” AgCl coating must be removed totally and the
electrode must be cleaned.
A damaged or weared AgCl coating will result in deviating measurements.
1.4.5.1 Before (re-)generating, the electrode must be cleaned
Clean the reference electrode before generating the AgCl-coating. When the electrode is very dirty (clearly visible
deposit/cover) it must be polished (for instance with 0.05 micron Al2O3powder).
When the electrode shows just a little surface deposits, it can be cleaned in two ways:
1. Put the Ag-electrode in a small water bath with water and soda and an aluminium foil on the bottom. The
silver part must be submerged in the solution.
Heating the water and adding some kitchen salt will speed up the cleaning process.
2. Electro-chemical cleaning:PlacetheAg-electrodeina3MKClor0.1MHClsolutionandanodizetheelectrode
till 15 minutes at a current density of 0.5 till 10 mA/cm2(according to Bailey (1980) 5 mA/cm2is
sufcient).
Connecting the Ag-electrode to the negative voltage - in relation to the counter electrode- will result in dis-
solvement of the Ag. Doing this for a short period (30 seconds) will clean the Ag-electrode.
If necessarry the electrode can be de-greased with aceton.
Always rinse with demi-water.
1.4.5.2 Generating an Ag-AgCl electrode
Method A: After cleaning, the Ag-electrode is placed in a concentrated (for example 5%) FeCl3solution.
The electrode stays in the solution for 10 minutes to create a good AgCl coating. After removing the
electrode from the solution a dull (mat) grey-black AgCl coating will be created. After 24 hours it will
darken.
Method B: Electro-chemical coating in KCl or HCl.
PlacetheverycleanAgelectrodeina3MKClor0.1MHClsolutionandanodizetheelectrodetill
15 minutes at a current density of 0.5 till 10 mA/cm2(according to Bailey (1980) 5 mA/cm2is
sufcient).
Connect the Ag-electrode to the positive voltage - in relation to the counter electrode (for
example Ag or Pt shaft) - this will result in a smooth, dull (mat) grey-black coating.
1.5 Brass electrode
The counter electrode in the current circuit is a brass rod. It has an integral coaxial signal cable. An outline of the
brasselectrodeisshowningure4onpage11.
1.5.1 Maintenance
An occasional light polish with ne sandpaper may be required to remove an oxide layer from
the brass surface. Avoid sharp bends in the signal cable. Do not use the glass bre pen for this
to avoid despositing brass on the platinum later.

6
2 Measurements
Check that the switch on the read-out unit is in the “OFF” position. Connect the signal cables from one, two or
three Pt-electrodes to the BNC input connectors on the read-out unit, numbered 1, 2and 3, as appropriate. Pre-
pare a place in the soil for the reference electrode and the brass electrode. A distance of about 2 metres between
theseelectrodesandthePt-electrodeswillusuallybefoundsatisfactoryintheeld,thoughitisbynomeanscriti-
cal.Inhorticultureadistanceofafewcentimetresmaybechosenwithoutanyproblem,dependingonthesizeof
the object. Connect a signal cable to the reference electrode. Bury the salt bridge and the brass electrode in the
top layer of the soil. In dry soils, it is often necessary to water the soil to improve the electrical contact between
these electrodes and the soil. Watering should be limited to the immediate vicinity of the salt bridge and the brass
electrode. Its effect must never extend to the Pt-electrodes. Connect the signal cables from the reference electrode
and the brass electrode to the corresponding inputs on the read-out unit. Allow 2 to 4 minutes for stabilisation.
2.1 Measurement of Redox
The ow of an electric current in the soil disturbs the Redox equilibrium. Re-equilibration after
an ODR measurement has been made may take between 15 minutes and several hours. So do
not switch to ODR measurement before having done redox measurements.
When the switch on the read-out unit is turned from the “OFF” position to the “Redox” position the Redox poten-
tial between the soil and the Pt-electrode at input no 1isdisplayedinmV.Ittakessometimewhentheelectrodes
have just been inserted into the soil before a steady reading can be obtained. This is often ten minutes, but in very
dry soils it may rise to a few hours. Therefore we suggest to take a reading after ten minutes, wait for another ten
minutesandtakeasecondreading.Whenthedifferenceislessthan20mV,thelatterreadingcanbetakenasthe
equilibrium value.
In contrast to ODR electrodes, Redox electrodes can be left longer in the soil without any
noticeable change of reading. After the rst equilibration no further time-delays are required.
It is important, though, that the part of the electrode structure that emerges from the soil is
perfectly dry (no rain or dew) and does not move (stabilise against wind).
The Pt-electrodes as supplied with the ODR meter can very well be used as Redox electrodes. A simpler design is
also feasible. In fact, a Pt wire or disc, mounted in an insulating rod and connected to a bare metal wire on top will
do, provided that no slit is left between the platinum and the insulation where water could penetrate. With the
read out unit in the Redox position and input no 1connected to a cable that has a crocodile clip at its free end, the
rapid measurement of a number of electrodes is possible.
2.2 Measurement of ODR
Turn the switch on the read-out unit to positions 1, 2or 3to read the ODR current at the corresponding elec-
trodes. The reading will rise to a high value and then continue to fall for some time, when the already dissolved
oxygen is reduced. It takes several minutes for the ODR measurement to reach an equilibrium. Therefore we sug-
gest to take a reading after about three minutes, wait for another three minutes and take a second reading to see
if an equilibrium has been reached yet.
Once the instrument is switched on, a current is maintained on all Pt-electrodes
connected to either input 1, input 2or input 3. Of course, only one Pt-electrode, determined by the position of the
switchisconnectedtothemeasuringcircuit.Thesteadyowofacurrentimplies,though,thatsubsequentmeas-
urementsaftertherstonerequirelesstime,typically20seconds.
One millivolt in the display corresponds to a current of one microampere. The voltage shown on the display is also
available as an analogue signal between contacts 1 and 5 of the output connector. To avoid an excessive current
drain from the batteries, the input impedance of any indicating or recording instrument should not be less than
ten kilo-ohms.

7
2.3 Checking the instrument
Checking general:
1. Check if the batteries are fresh
Check Redox measurements:
1. Switch instrument to Redox position
2. Apply voltage between “Pt electrodes 1 and “ref. electrode”
between0and2000mV.
Foraroughcheckyoumayusea1.5Voltpenlightbattery.
3. Displayreadingshouldbewithin3mVaccuracyofapplied
voltage.
Check ODR reference voltage:
Inthekitgoingwiththemeteryouwillndaresistancesolderedto
some wires. Use this simple tool for the next check.
1. Short circuit input brass electrode to input ref. electrode.
2. Connect resistance 820 ohms between input ref. electrode and
input PT electrodes 1
3. Switch instrument to position 1
4. At 820 ohms dsiplay reading should be 793
(+1ohm->-1mV:830ohms->reading783mV)
(-1ohm->+1mV:810ohms->reading803mV)
Check probe connections:
1. Check inputs and connections for corrosion and moisture (also on the instrument)
2. Check cables between middle pin connectors, resistance < 1 ohm
3. Check cables between outside connectors, resistance < 1 ohm
4. Check resistance reference electrode silver part (head without body) to middle pin connector, resistance < 1 ohm.
5. Check Pt electrode point resistance to middle pin connector, resistance < 1 ohm.
6. Check brass electrode to middle pin connector, resistance < 1ohm.
Check redox potential of electrodes:
Use a Zobell kalibration solution to check the electrodes for redox measurements. The measurement value must be
nearthecertiedvalueoftheZobellsolution(e.glabtemperature21°C,referenceelectrodeslledwith3MKCl,
measurementvaluemustbenear228mV).Stirrwithde-aerationalittlebitopen.
Which value will you get ?
This depends on the temperature and type of reference-electrode. The values are shown in the table on next page.
First you need to know the temperature of the (calibration) solution and the used reference temperature. Generally
aeldornormallabelectrodewillbeaPlatinum-silvercombinationtypeelectrodewith3MolKClliquid(saturated
with AgCl) as electrolyte.
Warning: The table is established using data from the Zobell-solution supplier (in Italics) and from the Austrian and
DIN standard. Missing but usefull data have been extrapolated and are marked with *.
Use the grey marked column for the 3 M KCl electrodes supplied by Eijkelkamp.
820 ohm

8
Temp
°C
Ag/AgCl
saturated
Ag/AgCl
(4M KCl)
Ag/AgCl
(3M KCl)
Ag/AgCl
1M KCl
Calomel Standard
hydrogen
Eh
Eref compared
to Ag/AgCl
(3M KCl)
-5 270mV 234.2
0 263.5 258* 226.0 482* 224*
5 257 251* 217.8 472* 221*
10 251 250.5 245 218 209.6 462 217
15 243 244 235 208 201.4 450 215
20 236 237.5 228 199 193.2 439 211
25 230 231 220 191 185.0 427 207
30 217 224.5 204 173 176.8 404 200
35 218 168.6
40 210 211.5 195 164 160.4 391 196
45 203 205 187 155 152.2 379 192
50 196 198.5 178 146 144.0 366 188
3 Storage, transport
Always use the protective covers provided when electrodes or the instrument will be trans-
ported.
Always clean the electrodes before storage.
The reference electrode may be stored in the bottle with KCI for some time, up to a few
months. When the instrument has to be transported over a long distance, or will be stored for
a long time, the reference electrode is best emptied, cleaned and dried. When it is to be used
again, the procedure as described under “1.4.2 Preparation for use” should be followed.
4 Specications
Range: Redox: 0to999mV
ODR: With the Pt-probe surface area of 2.37 * 10-5 m2(0.0000237 m2): 0 to 999 µA,
corresponding to an 02diffusion rate of 0 to 3500 micrograms/m2/s,
or 0 to 21 micrograms ·cm-2 ·min-1
Resolution: Redox: 1mV
ODR: 1 µA, corresponding to 3.5 micrograms/m2/s or 0.021 micrograms ·cm-2 ·min-1
Accuracy: Redox: ±3mV------------880299
ODR: ±3 µA
Climate: Operating temperature 0 °C to 50 °C, humidity 30 to 80 % relative humidity.
Output: Five-pinsubminiatureconnector,voltageof0to999mVbetweencontacts1and
5 for full range Redox or ODR signals.
Input impedance of indicator or recorder ≥10 kΩ.
Mating cable connector is supplied with the instrument.

9
Power: Four penlite (IEC-LR6) batteries; consumption 7.5 mA. Low battery-voltage level is indicated
in the upper left corner of the display by a battery symbol.
Battery replacement: Batteries can be reached through the lid at the bottom of the back of the read-out unit.
Mind the polarity when replacing batteries.
Dimensions: Carrying case 410 x 340 x 180 mm (L x W x H)
Pt-electrode length 800 mm, diameter 12 mm
Weight: Carrying case 4 kg
Pt-electrode 1 kg
Riverside auger 4 kg
Pt-electrode: Wire of platinum with 10 % iridium, diameter 1.2 mm, exposed length 6 mm, length 70cm
Reference electrode:Silver sheet 2 x 8 x 100 mm, ceramic cup, 100 ml PE plastic wide-neck bottle, probe
provided with a pressure release screw.
Brass electrode: Brass rod, diameter 8 mm, length 173 mm
Literature
An extensive discussion of the ins and outs of an ODR measurement has been given by D.S. McIntyre (The plati-
num micro-electrode method for soil aeration measurement, Adv. in Agron. 22: 235 - 283 (1970)).
Analysis with ion-selective electrodes. Second edition, 274 pp, Bailey, 1980.
A survey of principles and procedures of both ODR and REDOX measurements is given by Jan Glinski and Witold
Stepniewski in chapters 6 and 7 of Soil Aeration and Its Role for Plants, CRC Press, Inc., Boca Raton, Florida, USA,
1985.

10
Figure 1. Schematic diagram

11
Figure 2. Pt-electrode Figure 3. Reference electrode
Figure 4. Brass electrode
1
2
Nothinginthispublicationmaybereproducedand/ormadepublicbymeansofprint,photocopy,microlmoranyothermeanswithoutprevi-
ous written permission from Eijkelkamp Agrisearch Equipment.
Technicaldatacanbeamendedwithoutpriornotication.
Eijkelkamp Agrisearch Equipment is not responsible for (personal damage due to (improper) use of the product.
Eijkelkamp Agrisearch Equipment is interested in your reactions and remarks about its products and operating instructions.
Reference electrode and salt bridge
1 BNC connector
2 Electrode head with silver electrode
3 Pressure realease screw
4 O-ring
5 Obscuration
6 Porous cup
Pt-electrode: 1 Pt-Ir wire
2 BNC connector
1
2
3
4
6
5
Table of contents
Other EIJKELKAMP Measuring Instrument manuals

EIJKELKAMP
EIJKELKAMP Scuba User manual

EIJKELKAMP
EIJKELKAMP M1 User manual

EIJKELKAMP
EIJKELKAMP bbe AlgaeTorch 100 User manual

EIJKELKAMP
EIJKELKAMP Scuba Blue Green Algae Buoy User manual

EIJKELKAMP
EIJKELKAMP Aquameter Series User manual

EIJKELKAMP
EIJKELKAMP GX-8000 User manual

EIJKELKAMP
EIJKELKAMP 0865 User manual

EIJKELKAMP
EIJKELKAMP Aquaread Flowcell User manual

EIJKELKAMP
EIJKELKAMP QUICK DRAW 2900F1 User manual

EIJKELKAMP
EIJKELKAMP HD2 User manual