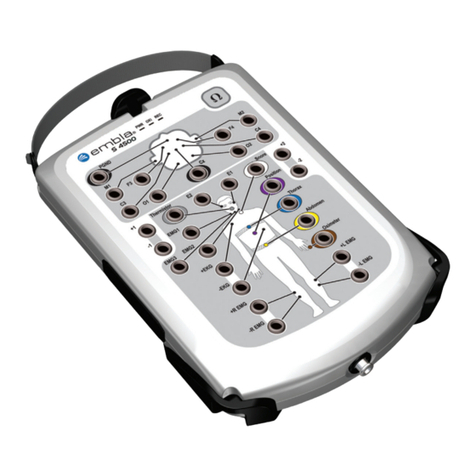
Universal–Single Use XactTrace™ Instructions
5
The belts do not need cleaning, as they should be discarded after use.
Warnings
See the list of warnings below.
Do not stretch the belts too tightly around the patient, as this may
cause discomfort. To prevent the belt from slipping during the
study, fix the position with medical tape.
The belts shall be worn over night clothes.
This product is for diagnostic purposes only, and is not to be used
as an apnea monitor.
Take care not to cut any cables when cutting the belts.
Do not use damaged sensors or accessories.
Be certain that cables or sensors do not encircle the patient’s
neck.
Do not use the equipment in a magnetic resonance imaging
(MRI) environment.
Do not use the device in an explosive environment—in other
words, in the presence of flammable liquids, such as aesthetic
mixture with air, or with oxygen or nitrous oxide.
Connect the XactTrace sensors to an electrically isolated input
only. Do not plug the cables into electrical outlets, as this could
result in serious electrical shock.
The device contains a battery; therefore, it must be disposed of
properly. Local, state, or national laws might prohibit disposal of
batteries in ordinary trash. Contact your local waste authority for
information regarding available recycling and disposable
options.
Portable and mobile radio frequency (RF) communications can
affect the performance of the device.