Embla XactTrace User manual

1
The Single Use XactTrace belt system is used during sleep studies to
acquire and transfer data related to thoracic and abdominal respiratory
effort to a compatible sleep recorder.
This document describes how to use the Single Use XactTrace belt
system and includes information on intended use, warnings and
cautions, system components, adjusting and attaching the belts, storage
and cleaning.
Intended Use
The Single Use XactTrace belt system measures respiratory effort to
assist in the diagnosis of sleep disorders or sleep related respiratory
disorders. The respiratory effort signals measured are processed to
provide electrical signals suitable for the inputs of physiological recording
equipment.
The intended environments are hospitals, institutions, sleep centers,
sleep clinics, or other test environments. The Single Use XactTrace belt
system is intended for diagnostic purposes only and is not intended to be
used as an apnea monitor. Use the Single Use XactTrace belt system
only under the direction and supervision of a physician or trained
technologist.
Warnings and Cautions
The belts are intended to be worn over the patient's nightclothes.
The belts are single use.
Do not stretch the belts too tightly around the patient as this may
cause discomfort

Single Use XactTrace™ Belt System User Instructions
2
Caution must be taken to ensure that cables do not encircle the
patient's neck. Special attention is needed in the case of
children.
Use the device only under the direction and supervision of a
physician or trained technologist.
Avoid all unnecessary contact with moisture when using the
device.
Do not use damaged belts, sensors or cables.
Caution: U.S. Federal law restricts this device to sale by, or on
the order of, a physician.
Portable and mobile RF communications can affect the
performance of the device.
Electrostatic discharges (ESD) may cause artifacts in the signal
from the device. A trained operator should be able to recognize
these artifacts easily. Avoid conditions where electrostatic
charge can build up due to low humidity and friction against
carpets, clothing, and sheets made from artificial fibers.
System Components
The system consists of a belt and a snap sensor with a cable that
connects directly to an input on the recording device. The system
converts changes in inductance to a digital signal that provides both
qualitative and quantitative information on respiratory effort.
Belt
Sensor

Single Use XactTrace™ Belt System User Instructions
3
Attaching the Single Use XactTrace System
The belts are intended to be worn over nightclothes and should fit the
patient snugly without being uncomfortably tight. Avoid all unnecessary
contact with moisture when using the Single Use XactTrace belt system.
Note: Do not use two thorax sensors or two abdomen
sensors in the same recording. Using two sensors of the
same type will cause interference between the sensors and
could result in poor signal quality.
Choosing a Belt Size
The Single Use XactTrace belts come in four sizes: Large, Medium,
Small and Pediatric. The length of the belt size can be adjusted to
ensure a good fit. The belts should fit the patient snugly without being
uncomfortable tight. Measure the patient’s circumference and use the
table below as a guideline. The belts are single use.
XactTrace Belt Sizes
Full Size
Reduced Size
cm
Inches
cm
Inches
Large
127 - 190
50 - 75
102 - 165
40 - 65
Medium
96 - 140
38 - 55
76 - 119
30 - 47
Small
61- 89
24 - 35
40 - 69
16 - 27
Pediatric
35 - 56
14 - 22
28 - 48
11 - 19
Single Use XactTrace™ Belt System User Instructions
4
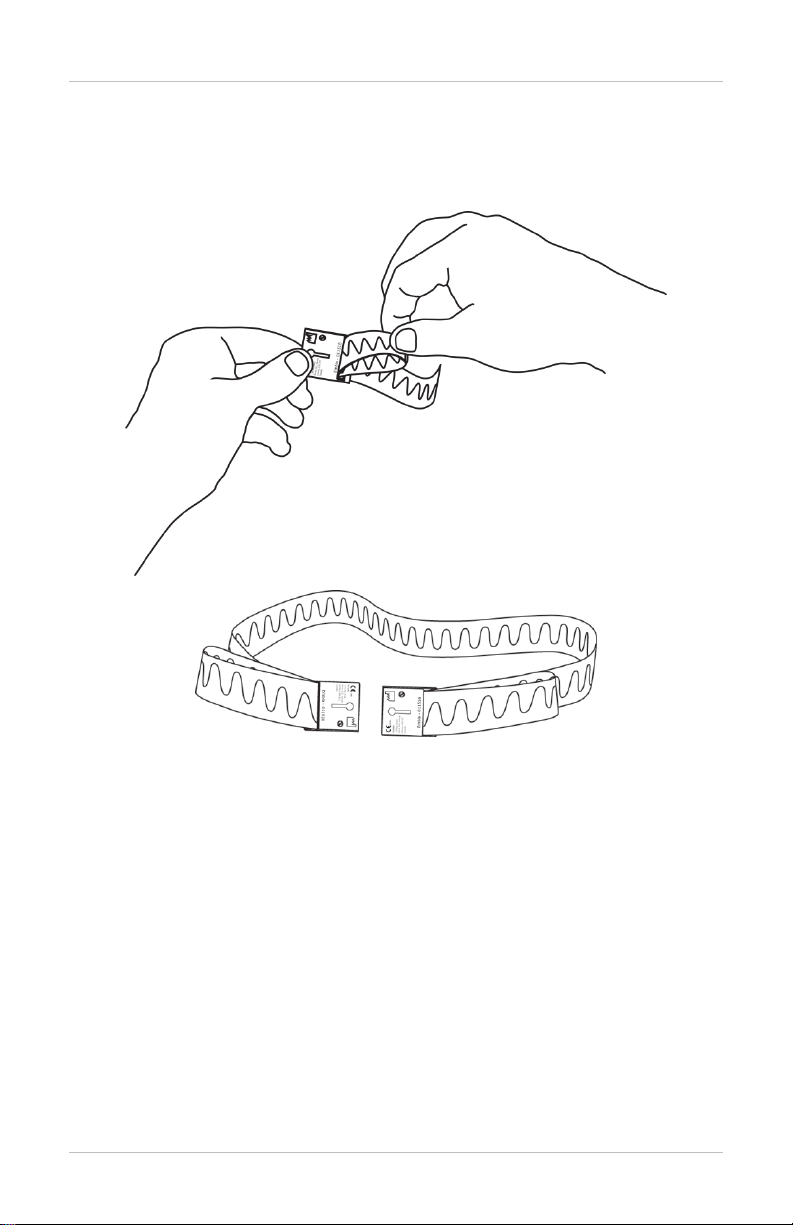
Adjusting the Belt Size
Adjust the belt by sliding the loop buckles on each end to maintain a
snug fit around the patient circumference.

Single Use XactTrace™ Belt System User Instructions
5
Attaching the Belts to the Patient
1. Encircle the belt around the patient chest and secure the snap
ends of the belt to the buckle with the “blue” connector as shown
in the figure below. The belt should fit snugly without being
uncomfortably tight.
2. Prepare the abdomen belt in the same manner, only this time, fit
the belt around the patients stomach and attached the snap ends
of the belt to the buckle with the “yellow” connector.
3. Connect the abdominal (yellow) and the thoracic (blue)
XactTrace connectors to the appropriate color-coded inputs on
the recording device.

Single Use XactTrace™ Belt System User Instructions
6
Storage
Proper storage extends the life of the Single
Use XactTrace belt system. To prevent
damage, do the following when storing the
sensor and belts between studies.
The belts are single use and should
not be used for more than one study.
Do not wrap the cable around the
sensor.
Cleaning
No part of the Single Use XactTrace belt system requires sterilization. If
desired, wipe the sensor and cables with a hospital grade cleaner that is
not corrosive to plastic or metal, and then dry with a clean, dry cloth. Do
not immerse the sensor in liquid and avoid contact of the cleaning
solution with the connectors.
Labeling Definitions
The manufacture date is adjacent to this symbol.
This symbol indicates that the item should not be reused and
is intended for a single-use only.
Maintenance
No special maintenance of the Single Use XactTrace belt system is
required.
Materials List
Category
Properties
Belt
Elastic belt W=25,WHITE,TERYLENE,WITH
WIRE,UL1571 #28,WHITE

Single Use XactTrace™ Belt System User Instructions
7
Category
Properties
Cable
CONN,2P+FLAT
CABLE,NONULN#24(46/0.08)*2C,BLACK
Snaps
SCREW,M3,THREAD L=3.2,L=8.8,D=2.95,D=4.6
Over-molding
PVC,45P,WHITE,UL94V-0
Warranty
Embla warrants the sensor to be free of defects in materials and
workmanship for 6 months from the date purchased. The sole liability of
Embla and our distributors is limited to replacement or repair of the
product at the option of Embla, with no charge for parts or labor if any
part is proven to be defective in workmanship, performance, or materials
during the warranty period. Under no circumstances shall Embla or our
distributors be liable for any loss of revenues or damage, direct,
consequential, or incidental, including loss of profit, property damage, or
personal injury arising from the use of, or the inability to use this product.
This warranty is intended for the original buyer and is in lieu of all other
warranties or previous agreements, expressed or implied. This warranty
is rendered void if the product is used for anything other than its intended
purpose or is subject to abuse, misuse, tampering, neglect or
unauthorized modifications. Use of this product constitutes acceptance of
this warranty in total.
Certifications
The Single Use XactTrace system is certified to carry the
CE mark. The CE mark is a declaration that the Single
Use XactTrace system is in compliance with the essential
requirements set forth by the European Union for medical
devices.
The Single Use XactTrace system is manufactured by Embla.

*013190*
REF: 013190, Rev. 05
Single Use XactTrace System User Instructions
Copyright © 2017 Natus Medical Incorporated. All rights reserved.
Printed in China.
013190, Rev. 05
All rights reserved. This manual contains proprietary information, which
is protected by copyright and may not be copied in whole or in part
except with the prior written permission of Natus Neurology Incorporated.
The copyright and the foregoing restrictions on the copyright use extend
to all media in which this information is preserved.
This copy of the User Manual shall be used only in accordance with the
conditions of sale of Natus Neurology Incorporated or its distributors.
Natus Neurology Incorporated makes no representations or warranties of
any kind whatsoever with respect to this document. Natus Neurology
Incorporated disclaims all liabilities for loss or damage arising out of the
possession, sale, or use of this document.
For assistance, please contact Embla Technical Support
(Ottawa.TechSupport@natus.com).
MANUFACTURER
EUROPEAN REPRESENTATIVE
Embla Systems
1 Hines Road, Suite 202
Kanata, ON K2K 3C7
Canada
Tel. : +1.613.254.8877
Fax : +1.613.270.0627
Natus Manufacturing Limited
IDA Business Park, Gort,
Co. Galway, Ireland
Tel. : +353-(0)91-64700
Fax : +353-(0)91-630050
TOLL-FREE IN NORTH AMERICA: 888 NO APNEA (888.662.7632)
www.natus.com Ottawa.T[email protected]
Table of contents
Other Embla Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual