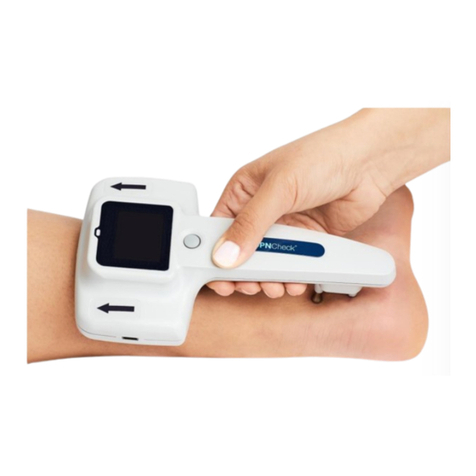
Device Use
Step 1: Lay the patient on an exam
table and prepare the testing area
with a Preparation Pad.
Step 8: Align the device on the lower calf by pushing down firmly on the foam.
The orange arrow closest to the midline of the calf should point towards the back
of the knee. When arrow is pointing properly, the inner edge of the biosensor will
then sit next to the midline (Achilles tendon).
Step 9: Press the button to start the
test. The light will blink with each
stimulation. Limb selected will be
displayed.
Tip: During test, maintain:
a.) Firm pressure on probes
b.) Firm pressure on biosensor
c.) Steady positioning
Step 6: Remove the backing from
the biosensor.
Step 7: Locate the patient’s outer
ankle bone to align the long probe
just behind it.
Tip: The anode (short probe, A) and
cathode (long probe, C) should be
aligned to the outer ankle bone, B.
The cathode should be adjacent to
the middle (central prominence) of the
ankle bone. The nerve is stimulated
only under the cathode.
Step 2: Power on the device.
The display will show and the
light will turn amber or red
when the battery needs to be replaced.
Step 5: Apply a small amount of
conductive gel to each probe.
The head of the probe should be
covered with gel.
Tip: Remove excess gel that may
lead to gel smearing between the
two probes.
Step 4: Set the leg to be tested. The
device display screen will blink with
the leg selected (l = left; r = right). To
switch the leg, hold the button down
for 1-2 seconds and the selection will
change to the opposite leg.
Tip: Whenever possible, test the
same leg in all patients.
Step 3: Fully insert the biosensor into
the port. The light will turn green once
the biosensor has been properly inserted.
Align to foam. The display will show
to set the leg to be tested.
Tip: Align the biosensor with the foam on
all sides; “REMOVE BACKING” label side
faces up.
Step 10: Maintain constant force
throughout the test. The test time
may vary per patient, but normally
lasts for 10-15 seconds. The results
will display once completed.
Testing Protocol
1. Testing a single leg is usually clinically sufficient.
• Whenever possible, the same leg should be tested first in all patients.
2. The test will provide a nerve conduction result the first time in most patients.
3. If the first test does not provide a result or to confirm the result, the test should be repeated.
• Pressing the test button again is usually all that is required (see Troubleshooting).
Tip: In certain circumstances it may be beneficial to confirm the results. Examples include:
- Confirm CV if amplitude is ≤ 4 µV
- Confirm undetectable response
- Confirm result inconsistent with clinical findings
- If the leg setting on the device was incorrect, then the test should be repeated
4. If the repeat test does not provide a result or for further confirmation of the results, the opposite leg
should be tested.
• The same biosensor may be used on both legs.
This protocol is intended only as a guide. It is the responsibility of the provider to determine the clinical necessity of nerve conduction testing.
••
Tip: Check for good contact on both
sides of the foam.
Test Results
Tip: The probes should be placed behind
but not over the outer ankle bone.
B
AC
•
•
Tip: Both the outer ankle bone (lateral malleolus) and
Achilles tendon should be easily accessible as shown
at left. Please reference the User Manual for alternative
patient positions.
The test area should be vigorously scrubbed with the
Preparation Pad provided.
Display Example Result Actions
Conduction Velocity – meters/second
Amplitude – microvolts*
Undetectable Response; no
Conduction Velocity displayed
Test Unsuccessful
40
4
0
Pn Pr Sn Lb
Ec oC Hd
--
|r
* Note: If the device displays an Amplitude > 0 but does not display a CV, the CV could not be reported on this individual.
Record Amplitude and interpret result.
To recall test results after the device powers down, press and hold the button for 3 seconds and you will see the results appear
on the display. Results will display for 10 seconds and then the device will power off. This mode can only be accessed when the
device is powered off.
Record and interpret result.
Record and interpret result.
Record and interpret result.
Note displayed code and refer to Troubleshooting
on back.