Contents
1Cleaning/ Disinfection – General Information. . . . . . . . . . . . . . . . . . . . . . . . 5
1.1 Cleaning Agents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.1.1 Approved Cleaning Agents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
1.1.2 Unapproved Cleaning Agents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
1.2 Disinfectants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
1.2.1 Approved Disinfectants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
1.2.2 Unapproved Disinfectants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
2Cleaning and Disinfecting the Device Components . . . . . . . . . . . . . . . . . . . 7
2.1 Device Surfaces (Plastic / Metal) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2.2 Handlebar / Seat (Saddle) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2.3 Pedals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2.4 Displays of Control Terminals. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
2.5 Upholstery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
2.6 Blood Pressure Cu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
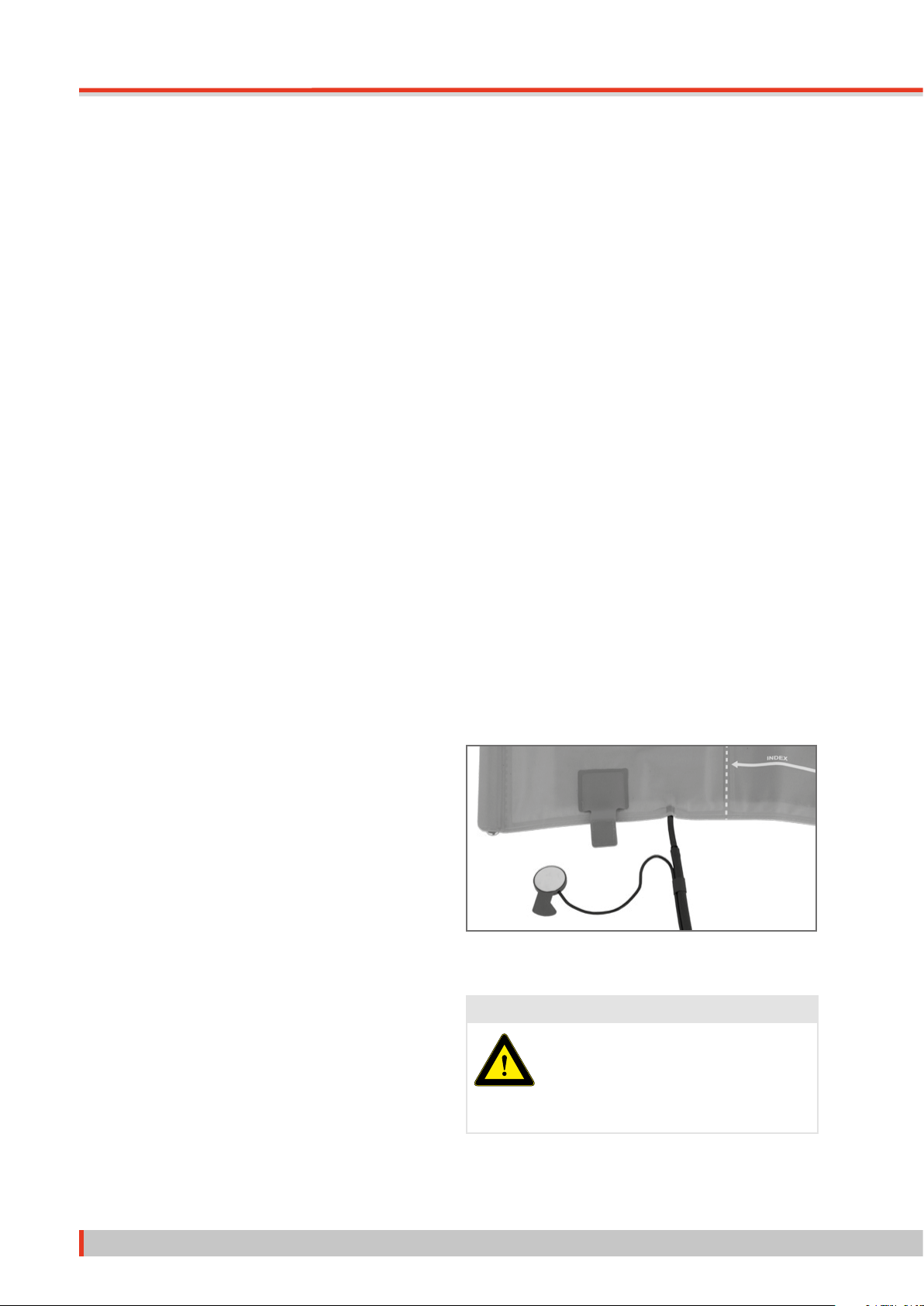
2.6.1 Removing the Microphone . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
2.6.2 Cleaning. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
2.6.3 Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
2.6.4 Inserting the Microphone. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
2.6.5 Inspection and Functional Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
2.7 ECG Electrodes / Suction Leads. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
2.7.1 Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
2.7.2 Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
2.8 SoftTip SpO2 Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
2.8.1 Aids for Cleaning / Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
2.8.2 Manual Cleaning and Disinfection of the SoftTip Sensors. . . . . . . . . . . . . . 12
2.8.3 Cleaning the Cables. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
2.8.4 Inspection and Functional Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
2.9 Ear Clip . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Cleaning and Disinfecting ergoline Medical Devices 3
Contents