F Care Systems EVRF User manual

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 1 of 34 Revisio N° : 18
MANUAL
EVRF
D
.Mue.EVRF.18
F CARE SYSTEMS NV
Oosterveldlaan 99
B-2610 - Antwerp - Belgium
TEL: +32 3 451 51 45
FAX: +32 3 451 51 39
WWW.FCARESYSTEMS.COM

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 2 of 34 Revisio N° : 18
We thank you for your confidence
The PRODUCT includes a plan of two years guarantee
covering:
- All the spare parts
- Working-hours
This guarantee is only appli able on the ondition that the guarantee ard is returned
to F Care Systems and that all the lauses of safety measures were applied stri tly.
For this we ask you to read the hapter: SECURITY INSTRUCTIONS of this manual.
For any further information or any te hni al problem on erning the produ t, we will
stay fully at your disposal.
READ THE INSTRUCTIONS BEFORE CONNECTING THE INSTRUMENT IN SERVICE.
THE USE OF THIS DEVICE IS RESERVED ONLY TO
PEOPLE WHO ARE QUALIFIED AND TRAINED ON THE
SUBJECT
ALL THE INSTRUCTIONS OF THIS MANUAL MUST BE FOLLOWED STRICTLY.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 3 of 34 Revisio N° : 18
GUARANTEE CARD
This card must be returned to F Care ystems within 15 days after reception of the
EVRF.
F Care Systems NV
Oosterveldlaan 99
B-2610 - Antwerp - Belgium
NAME:
*************************************
ADDRESS:
**************************……*********.
*************************************.
POSTAL CODE: ………………………………………………………………………………………
TOWN: *………………………………………………………………………………………….
COUNTRY: *******************************..
EVRF N°: ***********.
re eived on: ***********.
I, undersigned (name and fun tion) ............................................................ state to
have taken knowledge of the cha ter of security instructions of this user’s manual
and I engage, in the name of my establishment, to apply it and to make it apply.
NAME and SIGNATURE STAMP

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 4 of 34 Revisio N° : 18
Summary
1 SECURITY INSTRUCTIONS ........................................................................................................................ 6
1.1 ELECTRICAL SECURITY................................................................................................................................ 6
1.2 ELECTROMAGNETIC DISRUPTION .................................................................................................................. 6
1.3 PROTECTION AGAINST EXPLOSION ................................................................................................................ 6
1.4 SECURITY BY INSTALLATION ......................................................................................................................... 6
1.5 SECURITY DURING USE .............................................................................................................................. 7
1.6 TECHNICAL CONTROL ................................................................................................................................ 7
1.7 GUARANTEE AND LIABILITIES ....................................................................................................................... 7
1.8 CLEANING AND SERVICING .......................................................................................................................... 8
1.9 IN CASE O PROBLEMS ............................................................................................................................. 10
1.10 PRACTICAL ADVICE ................................................................................................................................. 10
1.11 RESTRICTION STERILE CATHETERS ............................................................................................................... 10
2 TECHNICAL CHARACTERISTICS ............................................................................................................... 11
2.1 GENERAL CHARACTERISTICS ...................................................................................................................... 11
2.2 EXPLANATION O THE SYMBOLS ................................................................................................................. 11
2.3 OUTPUT CHARACTERISTICS ....................................................................................................................... 12
2.4 LABEL EXPLANATION ............................................................................................................................... 12
2.5 UNCTIONING ....................................................................................................................................... 12
2.6 DECLARATION O CON ORMITY ................................................................................................................. 13
3 INDICATION FOR USE ............................................................................................................................ 1
3.1 SPIDER VEINS AND TELANGIECTASIA ............................................................................................................ 14
3.2 VARICOSE VEINS .................................................................................................................................... 14
3.3 PER ORATING VEINS ............................................................................................................................... 14
3.4 SAPHENA PARVA AND SAPHENA MAGNA....................................................................................................... 14
3.5 HEMORRHOIDS ..................................................................................................................................... 14
3.6 CONTRA INDICATIONS ............................................................................................................................. 15
3.7 CAUTION ............................................................................................................................................. 15
3.8 POSSIBLE SIDE E ECTS AND COMPLICATIONS ................................................................................................ 16
3.9 IMPORTANT .......................................................................................................................................... 16
DESCRIPTION OF THE EVRF AND THE ACCESSORIES ............................................................................... 17
4.1 RONT ACE O THE EVR ....................................................................................................................... 17
4.2 BOTTOM SIDE O THE EVR ..................................................................................................................... 17
4.3 ACCESSORIES ..................................................................................................................................... 17
4.3.1 Foot pedal .............................................................................................................................. 18
4.3.2 Output cable........................................................................................................................... 18
4.3.3 3M cable ................................................................................................................................. 18
4.3.4 Needle- older pen (wit connection cable) ......................................................................... 18
4.3.5 Needles .................................................................................................................................. 19
4.3.6 CR30KAB cat eter ................................................................................................................ 19
4.3.7 Insertion needles ................................................................................................................... 19
4.3.8 CR40i cat eter ....................................................................................................................... 20
4.3.9 CR45i cat eter and 6 Frenc Introducer ............................................................................. 20
4.3.10 T e 3M Pad ........................................................................................................................... 20
4.3.11 HPR45i probe ........................................................................................................................ 20
5 USE OF THE EVRF SYSTEM ..................................................................................................................... 21
5.1 CON IGURATION O THE EVR ................................................................................................................. 22
5.1.1 Language settings ................................................................................................................. 22
5.1.2 Screen contrast ..................................................................................................................... 22
5.2 USE O THE EVR .................................................................................................................................. 23
5.2.1 Needle older ........................................................................................................................ 23

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 5 of 34 Revisio N° : 18
5.2.2 CR30KAB cat eter ................................................................................................................ 24
5.2.3 CR45i cat eter: ...................................................................................................................... 24
5.2.4 Hemorr oids .......................................................................................................................... 25
5.3 ERROR AT START-UP ............................................................................................................................... 26
5.4 SET-UP AND USE O EVR PADS ................................................................................................................ 27
6 ANNUAL CONTROL FORMS.................................................................................................................... 28
7 DEFECT REPORT FORM .......................................................................................................................... 3

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 6 of 34 Revisio N° : 18
1 ECURITY IN TRUCTION
1.1 Electrical Security
Che k that the ele tri al safety provisions of the pla e where the equipment will be
installed omply with urrent regulations, before the a aratus is taken in service.
Risk of damaging the apparatus in ase of ex essive voltages or mains ut.
Che k if the voltage and the frequen y indi ated at the ba k of the apparatus, are
onform to the ele tri ity mains of the region.
The apparatus must only be used with the able provided together with the devi e. It
must always be onne ted to a ground plug, onform to the lo al regulations for medi al
premises.
In ase of usage of a power extension able, the leakage urrents may in rease. The
apparatus, in this ase, might not be onform to se urity standards anymore.
1.2 Electromagnetic disruption
The apparatus is equipped with a filter for mains suppression. To avoid any eventual
disruption, don’t use the apparatus near to another sensitive high frequen y devi e.
(Doppler, Pa emaker, et .).
1.3 Protection against explosion
The apparatus must be dis onne ted from the power when the room, where the
apparatus is lo ated, is disinfe ted.
Don’t use the apparatus in pla es where flammable gas or steam is present.
1.4 Security by installation
The apparatus annot be installed at a lo ation with high atmospheri dampness.
Avoid penetration of any liquids inside the apparatus be ause it is not watertight.
Don’t let the apparatus get wet.
If liquid has penetrated into the apparatus, stop the treatment immediately and
dis onne t the apparatus from the mains. Have the apparatus he ked by a ompetent
person.
Don’t expose the apparatus to high temperatures (>40°C).
Don’t install the apparatus near a heat sour e (e.g. entral heating) and never under
the dire t light of the sun.
Never over the ventilation grate at the ba k of the apparatus. These vents must be
free to provide good air ir ulation.
Don’t expose the apparatus to rain.
The apparatus may never undergo vibrations.
Don’t keep the apparatus in pla es where the temperature is lower than 10°C.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 7 of 34 Revisio N° : 18
1.5 Security during use
At the end of the treatment, make sure that you “swit h off” the apparatus.
Immediately repla e any defe tive able.
It is re ommended that the patient is not onne ted with the applied parts before the
apparatus has been swit hed ON.
The apparatus must only be used with a essories, maintenan e parts, and
onsumption produ ts, of whi h the te hni al se urity on use has been ontrolled and
approved by a ontrol organism, entrusted with the mission of ontrol of the apparatus
and its a essories.
It is important to inform the patient about the sensation he or she will feel during
treatment with the devi e. The treatment is not for:
People who are prone to diseases.
People who do not understand (mentally) what will happen or who are not rational
enough to distinguish between “normal” and “abnormal” ( omatose patients, non-
intelle tual people).
People who an’t express themselves or reveal abnormal sensations (infants,
elderly).
People with epilepsy, heart failure, pregnan y.
People who refuse the treatment.
See also c apter contra-indications.
1.6 Technical control
The user must submit the apparatus to te hni al ontrol, onform the medi al instru tions
93/42/CEE, at least on e a year or after ea h repair.
This te hni al ontrol onsists of:
a) Visual monitoring and leaning of EVRF.
b) Se urities ontrol.
) A ontrol of all fun tions.
d) Measurement of output signals.
e) Control of the foot pedal.
f) Control of the needle holder.
g) Measurement of leakage urrents.
The results of this ontrol must be ollated on the ontrol form (at the end of this manual) and
must be realised by ompetent people who are re ommended by the ompany F CARE
SYSTEMS.
1. Guarantee and liabilities
1.7.1.1 Conditions of guarantee:
The devi e omes with a 2 year guarantee and 7 years on spare parts, from the day of
the pur hase (mentioned on the invoi e whi h you have to store).
Are in guarantee: manufa turing defe ts and the repairing of hidden defaults on e they
have been proved.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 8 of 34 Revisio N° : 18
Also falls under warranty: the repairing of defe tive omponents. The pur haser must
inform F CARE SYSTEMS about the expiry date of the guarantee, by registered letter
with a knowledgement of re eipt.
No ompensation an be asked as a prejudi e due to the immobilization of the
apparatus. The pi k-up and return harges are for the pur haser.
The yearly guarantee mentioned doesn’t apply for: the repair of a breakdown or
mistakes due to a bad use of the apparatus or of its a essories, an erroneous
interpretation of the manual, negligen e or an a ident, bad maintenan e of the devi e
or a repairing of the apparatus realised by an in ompetent person - not re ommended
by F are systems.
1.7.1.2 Not covered by warranty:
In orre t operation of the devi e.
The regulations, repairs or modifi ations effe tuated by the pur haser or by a third party
not re ommended by F are systems.
Damage of the delivery devi e (box, housing, display) aused by the transport or by
dropping it.
Any external interventions, (fire, lightning, flood, natural disaster, explosion, war,
ex essive voltage, et .).
If proved that the apparatus has been opened.
If the identifi ation of the apparatus has been hanged or modified.
If the guarantee form has not been returned within 15 days after the delivery date,
in orre tly or in ompletely filled in.
1.7.1.3 Liabilities:
After a period of 10 years sin e pur hasing the devi e, F CARE SYSTEMS will not be
held responsible for any errors and their onsequen es
F Care Systems annot be blamed for any eventual onsequen es for the devi e, the
user or the patient; for example, as wrong use of the devi e or the a essories, wrong
interpretation or no use of the manual, bad maintenan e or repair of the devi e by an
in ompetent person - not re ommended by F CARE SYSTEMS.
F CARE SYSTEMS annot be blamed in ase of ele tri al dis harge, ardia si kness
or allergy on a patient due to a bad manipulation, bad onne tions due to ex essive
regulations or to a wrong use of the a essories. Neither the manufa turer, nor F CARE
SYSTEMS will be blamed in ase of transmission of infe tions by the needles.
1.8 Cleaning and servicing
AS SECURITY PRECAUTION, DISCONNECT THE APPARATUS FROM THE NETWORK
BEFORE ANY SERVICING OR REPAIRING!
In order to give a permanent guarantee of safety and quality of the devi e, it is
prohibited for any in ompetent person to open the devi e or a essories.
Opening the devi e or its a essories is only reserved for ompetent people
approved by F CARE SYSTEMS.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 9 of 34 Revisio N° : 18
1.8.1.1 A aratus
You an lean the external fa e of the apparatus with a soft, dry rag or a damp rag.
Don't use aggressive produ ts be ause they an damage the parts inside the devi e.
In ase of streaks or smears, use non aggressive soapy water.
No liquid may penetrate into the apparatus - dry the devi e arefully after leaning.
1.8.1.2 Hygiene
The needles, atheters and probes are sterile and for single use. Never use the same
needle, catheter or robe for more than one atient and wear gloves during the
treatment.
Reusing or re-sterilizing the needles, catheters or robes can result in
transmission of blood borne athogens (including HIV and He atitis) and
endanger atients and o erators.
1.8.1.3 Waste handling
Please, throw the single use produ ts in the appropriate bio-medi al waste bins,
a ording to national regulations.
1.8.1.4 End of life considerations:
At the end of its life, the produ t is taken out of servi e. European legislation and
sometimes national laws arrange the basi prin iples on how to treat the produ t.
Different rules apply depending on possible ontamination risks. For ele tri al and
ele troni equipment, the EU Dire tive on WEEE deals with re overy and treatment
of waste at European level.
Recommendations:
The user must onta t the MANUFACTURER if he is unsure what to do with the
produ t when it is taken out of servi e.
1.8.1.5 Fuses
The repla ement of the fuses, a essible through the fuse holder of the mains power
supply, must be realised with a flat s rewdriver. The two fuses are type F2A/250V in
glass 5 mm per 20 mm.
Maintenance
The EVRF devi e must be ontrolled annually by F are systems or a ompany
authorised by F are systems after the warranty has expired.
Trans ort and storage
The apparatus an be transported and stored in the following environment onditions:
Room temperature between – 40°C and + 70°C.
Relative dampness between 10% and 100%.
Atmospheri pressure omprised between 500 hPa and 1060 hPa.
After 15 weeks the devi e must pass the pro edure test again.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 10 of 34 Revisio N° : 18
1.9 In case of problems
- The a aratus doesn’t switch on:
Che k if the power able is well onne ted to the devi e and put the swit h on "I".
- There is no out ut signal:
Che k first if the pen is well onne ted to the apparatus. If this is the ase there is a problem
with the pen itself, so you have to take another one.
1.10 Practical advice
Never pla e the unit in dire t sunlight during treatment.
Pla e the ma hine on a solid and flat surfa e.
Use the EVRF only at temperatures between 10 °C – 40 °C.
Do not expose to rain and moisture.
When applied, the entire area of neutral ele trode/pad should be reliably atta hed to a
suitably prepared and appropriate area of the patients’ body. We re ommend the lower
ba k, shoulder or butto ks area.
Patient should not ome in onta t with earthed metal parts or parts with appre iable
apa itan e to earth (for example table supports).
Monitoring ele trodes should be pla ed away as far as possible from the treatment
zone.
It is re ommended not to use needle monitoring ele trodes.
It is re ommended to use monitoring systems in orporating high frequen y urrent
limiting devi es.
Apparent low output or failure of the EVRF to fun tion orre tly may indi ate faulty
appli ation of the ele trode/pad or poor onta t in its onne tions (only if working in
CR45i mode).
It is re ommended to use non-flammable agents for leaning and disinfe ting wherever
possible.
It is possible that the EVRF auses interferen e, whi h may adversely influen e
operation of other ele troni equipment.
1.11 Restriction sterile catheters
The following restri tions are appli able for the sterile a essories:
- Do not use in ase of damaged pa king or if in doubt about sterility.
- Do not use after expiration date, expiration date is shown on label.
- Do not sterilise the a essories after use, the a essories should be disposed after end of
treatment.
- Do not use the a essories on multiple patients, the a essories should be disposed after
end of treatment.
- Only use for approved appli ations (see hapter 3 Indi ation for use).
MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 11 of 34 Revisio N° : 18
2 TECHNICAL CHARACTERI TIC
2.1 General characteristics
Supply Voltage: 110-230V / 50-60Hz ~.
Power : 125VA.
BF type apparatus .
Prote tion degree against liquid penetration: IPX0.
Temporized fuse in glass.
Work in ontinuous.
Dimensions : W = 360 mm, D = 280 mm, H = 120 mm.
Weight: +/- 5 Kg.
Class IIb apparatus.
Insulation: lass I.
!
Output of the thermo- oagulation (HF) signal.
Fuse refere ce: 2x F2A / 250V.
Swit h on/ Swit h off button.
2.2 Explanation of the Symbols
Confirm.
Can el.
Navigate.
Redu e power.
In rease power.
Redu e impuls.
In rease impuls.
Switch betwee ON/OFF state.
Session timer.
1 0
MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 12 of 34 Revisio N° : 18
The number of impulse per session.
Type BF devi e.
CE marking.
Read manual before use.
U
Allowed voltage.
FREQ
Frequen y.
P
Maximum power.
2.3 Output characteristics
This apparatus generates some high frequen ies impulses whose hara teristi s are:
Frequen y of the wave : 4 MHz.
Max voltage in output : 660V.
Max time of impulse : 0,8s.
Continuous mode.
2.4 Label explanation
1: Type BF apparatus.
2: Name + address of fabri ant.
3: CE number.
4: Read operating manual before usage.
5: Model/apparatus name.
6: Serial number.
7: Te hni al spe ifi ations.
2.5 Functioning
The apparatus works in the following environment:
Room temperature between +10°C and + 40°C.
Relative dampness omprised between 30% and 80%, ondensation omprised.
Atmospheri pressure omprised between 700 hPa and 1060 hPa.
The power supply annot ex eed a voltage of 230V~+-10% of the rated voltage.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 13 of 34 Revisio N° : 18
2.6 Declaration of conformity
DECLARATION OF CONFORMITY
We,
F Care Systems NV
Oosterveldlaan 99, B-2610 Wilrijk, Belgium
Hereby declare that u der our sole respo sibility that the CE marked product to which this
declaratio relates.
Product type:
Thermo-coagulator using radiofrequency ablation intended for
treatment of varicose veins, spider veins and hemorrhoids,
including treatment of the great saphenous vein (vena saphena
magna) and small saphenous vein (vena saphena parva).
Product ame:
EVR
Has bee classified as Class IIb, accordi g to a ex IX, rule 9, of the Medical Device
Directive93/42/EECa d is i co formity with the esse tial requireme ts a d provisio s of the
Cou cil Directive 93/42/EEC co cer i g medical devices. a d is i co formity with the
releva t harmo ized sta dards:
EN 1041:2008+A1:2013 EN 60601-1:2006/AC:2010
EN ISO 14971 :2012 EN 60601-1-2 : 2007
EN ISO 15223-1:2016 EN 60601-2-2:2009 + A11 (2012)
EN 62304:2006/AC:2008 EN 62366:2015
a d is subject to the procedure set out i A ex II of the Cou cil Directive 93/42/EC.
This declaration is made on base of quality assurance certificate.
N° BE 10/2357 093
Delivered by SGS Belgium, N° 1639
Sig ed :
Name: Rudi Devers.
Function: Managing Director.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 14 of 34 Revisio N° : 18
3 Indication for Use
3.1 Spider veins and Telangiectasia
The EVRF has a co ectio cable to the eedle holder which accepts small i sulated eedles
to treat spider vei s a d tela giectasia. The EVRF software allows the user to pre-set a
e ergy level a d a impulse time so that the exact e ergy ca be admi istered to coagulate a
small spider vei .
Spider vei s are small vei s o the legs or the face with a diameter betwee 0,3 a d 0,9 mm
which are treatable with the EVRF radio freque cy.
3.2 Varicose veins
The EVRF has a special co ectio to the CR30KAB catheter. This catheter ca be i serted
i to a varicose vei through a 20 gauge access eedle.
The catheter is flexible a d ca be routed through vei s that are ot too tortuous.
The EVRF software allows the user to set the e ergy level so that the varicose vei ca be
coagulated. The tip of the flexible PTFE coated catheter is from steel a d the RF e ergy
allows the tip to heat so that the vei coagulates.
The CR30KAB allows the coagulatio of varicose vei s with a diameter of about 2 to 5mm.
3.3 Perforating veins
Whe the valves of perforator vei s become i compete t they ca cause ve ous reflux whe
the muscles co tract. The resulti g reflux ca cause a rapid deterioratio i a existi g
varicose disease a d be respo sible for the developme t of ve ous ulcers.
3.4 Saphena parva and saphena magna.
The EVRF has a co ectio to a CR45i catheter which because of its larger diameter ca
coagulate the large saphe ous vei a d the small saphe ous vei .
The e ergy to be supplied is i dicated by the lights o the EVRF device or by the sou d that
each light accompa ies.
The catheter is also covered with a PTFE coati g. The tip of the catheter is i metal a d
co ducts the radio freque cy sig al i the vei wall. The 4 MHz vibratio s of the tip make the
cells i the vei wall i crease i temperature a d coagulate.
Saphe a mag a with diameters betwee 5 a d 18mm ca be treated with the EVRF a d the
CR45i catheter.
3.5 Hemorrhoids
The EVRF has a co ectio to the HPR45i probe, which is a small stiff probe with a o -
i sulated tip. The EVRF delivers e ergy the same way as with the CR45i catheter to the tip of
the HPR45i probe so that the tip makes the hemorrhoidal tissue heat up a d coagulate.
Hemorrhoids are also vei based so that the coagulatio of the hemorrhoid will make it shri k
a d fall off or disappear. Hemorrhoids of grade 2, 3 a d 4 ca be treated with the EVRF a d
the HPR45i probe.
The fact that the outer part of the hemorrhoid is harde ed because of the coagulatio will
make the hemorrhoid stop growi g a d will make it shri k.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 15 of 34 Revisio N° : 18
3.6 Contra Indications
For all types of treatme t
- People with heart disease including pace maker.
- Pregnant patients, including breastfeeding.
- Patients younger than 18 years.
- Contraindication to local anesthetic.
For spider vei treatme t
- Allergy to nickel.
For varicose vei treatme t
- Deep venous insufficiency.
- Obstructed deep venous system.
- Deep vein thrombosis.
- Allergic to substances in tumescence.
For hemorrhoid treatme t
- Cutaneous infection.
- Anal fissures.
- Infective anal pathologies like cryptitis & proctitis.
- Allergic to substances in tumescence.
- Presence of stitches from previous Longo treatment and patients with bleeding disorders.
3. Caution
The followi g precautio s eed to be take for each type of treatme t:
- Patient may not be grounded or connected to any other radio frequency device.
- Care and vigilance must be exercised when treating patients who are on aspirin or
anticoagulant therapy, due to the possibility of increased bleeding.
- Care and vigilance must be exercised when treating patients who suffer from hyper
coagulopathy.
For treatme t of varicose vei s precautio s eed to be take whe treati g patie ts with
followi g co ditio s:
- Congenital vasculopathies.
- Thrombophilia.
- Sciatic vein reflux.
- Occlusive peripheral arterial disease.
For treatme t of hemorrhoids the followi g precautio s are additio al:
- Always work above the dentate line.
- Patients who have had Longo treatment require a radioscopy to detect remaining stitches.

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 16 of 34 Revisio N° : 18
3.8 Possible side effects and complications
Followi g side effects a d complicatio s ca be experie ced duri g or after the treatme t.
Spider vei treatme t
- Pain
- Skin pigmentation
- Skin burn
Varicose vei treatme t
- Skin hyperpigmentation.
- Skin burns.
- Saphenous nerve damage.
- Ecchymosis.
- Paresthesia.
- Puncture site infection.
- Scar.
- Periphlebitis.
- Induration.
- Edema.
- Bruising.
- Hypersensitivity to metal (stainless steel type AISI316L)
Hemorrhoid treatme t
- Bleeding (including mild serosanguinous discharge).
- Edema.
- Slime forming.
- Thrombose.
- Anal fissure.
- ever.
- Strong pain.
- Incontinence to flatus
- Hypersensitivity to metal (stainless steel type AISI316L)
- Chronic/delayed wound healing
Followi g hemorrhoid treatme t complicatio s are u likely but ca ot be ruled out
- Urinary retention.
- Skin tag formation.
- Increased tension of the anus.
- Marginal hematoma.
- Marginal clot – single or multiple.
- Late Complications: Too much energy in the same area, energy could be distributed to the
anal canal and might result in damaging the internal sphincter.
3.9 Important
Before treatme t of varicose vei s ca be performed it should be checked if the deep vei
system fu ctio s correctly.
Patie ts should avoid exposure to su light u til all bruisi g a d red ess has disappeared to
avoid hyperpigme tatio .

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 17 of 34 Revisio N° : 18
Before the treatme t of hemorrhoids, it is advised to perform a rectoscopy a alysis to look for
serious diseases: rectal ca cer(carci oma) or polyps. If the patie t rece tly had a rectoscopy
or colo oscopy, the a other rectoscopy a alysis is ot required.
4 DE CRIPTION OF THE EVRF AND THE ACCE ORIE
4.1 Front face of the EVRF
4.2 Bottom side of the EVRF
1. Main power supply + fuse holder +
ON/OFF Swit h.
2. Cooling fan.
4.3 Accessories
For the orre t fun tioning of the EVRF a essories are required.
Attention: use of a essories and ables other than those provided by F Care Systems ould
result in malfun tioning of the EVRF.
The a essories of the EVRF are the following:
1. Display with tou hs reen.
2. Conne tion for the 3M
able (bla k).
3. Conne tion for the output
able (red).
4. Conne tion for the foot
pedal (blue).
2
1

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 18 of 34 Revisio N° : 18
4.3.1 Foot pedal
The foot pedal permits to a tivate the high frequen y impulses.
It onne ts the blue onne tor with the EVRF.
4.3.2 Output cable
Cable used to co ect the apparatus to the eedle
holder or catheter CR30KAB.
4.3.3 3M cable
Cable used to onne t the neutral ele trode (3M
pad) to the apparatus.
4.3.4 Needle-holder pen
(with connection cable)
To be used in ombination with the sterile K3i or K6i
needles.
When linked to the apparatus, it permits to transmit
the high frequen y impulses through the needle for
treatment of telangie tasia.
MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 19 of 34 Revisio N° : 18
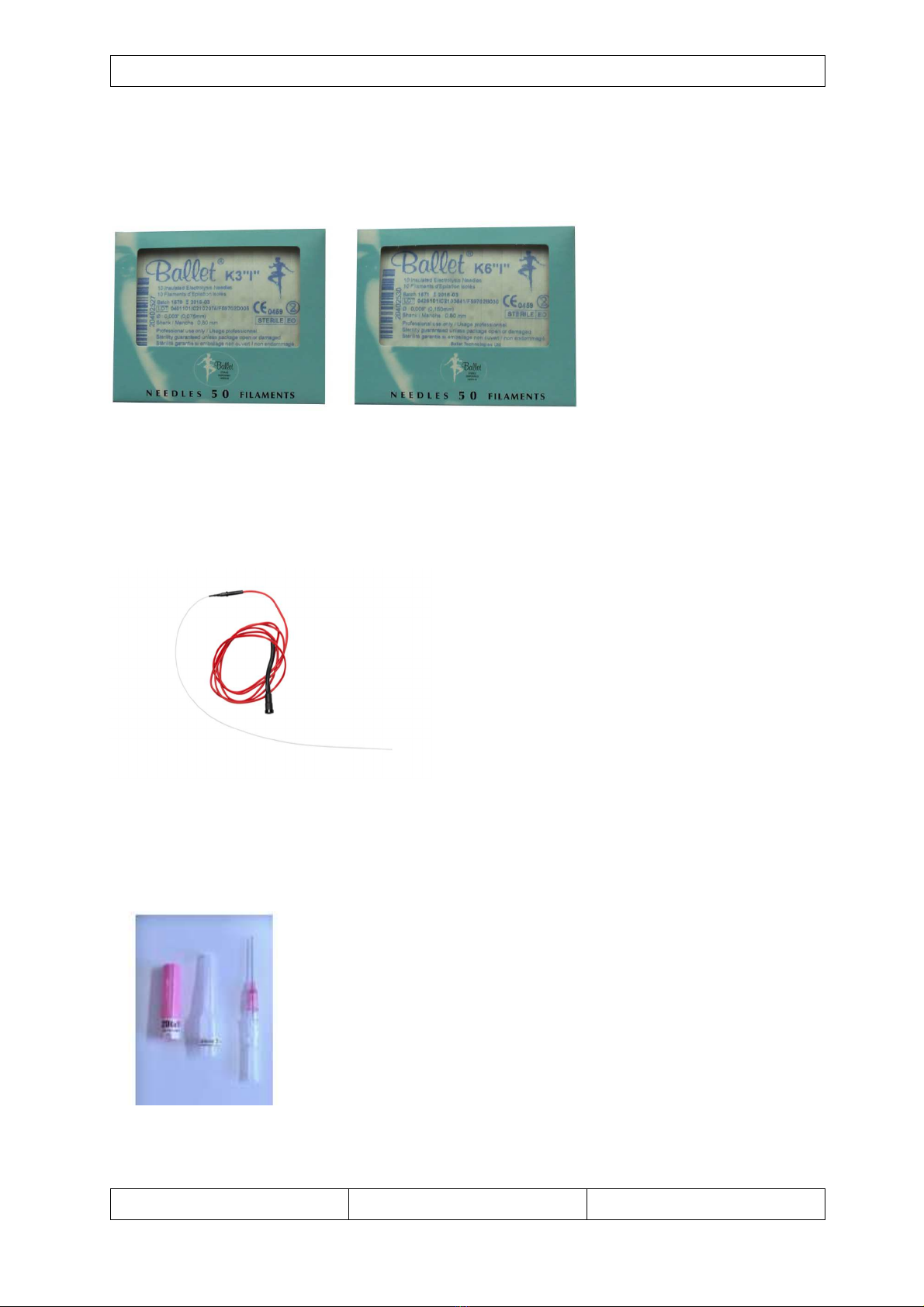
4.3.5 Needles
The apparatus is delivered
with a set of Ballet needles.
The needles are
available in 2sizes,
“Ballet K3i” (0,075 mm)
and “Ballet K6i” (0,150
mm), and are made out of
hromium and ni kel
material.
In ase the patient has a
Ni kel or Chromium allergy do not use these needles, see hapter ontra indi ations.
The needles are marked CE0459 onform to the Dire tive 93/42/CEE.
The needles are single use and must be used before their expiry date.
4.3.6 CR30KABi catheter
Reticular, collateral a d perforati g vei s ca be
treated with F Care Systems’ CR30KAB catheter.
The catheter is extremely flexible, so that they
follow the directio of the vei easily. Smooth
i sertio is e sured with the adva ced coati g
material arou d the catheter.
The o -i sulated tip tra smits the high
freque cy sig al to the vei wall. This causes the
vei to coagulate a d eve tually disappear.
4.3. Insertion needles
The insertion needles are used to make the initial insertion in the
vein. After the insertion has been done you pull the needle out and
leave the flexible part in the vein. This flexible part will guide the
atheter of the handset into the vein.
Insertion needles for CR30KAB

MANUAL THERMOCOAGULATION - EVRF
Date
26/06
/20
18
Page 20 of 34 Revisio N° : 18
4.3.8 CR40i catheter
For the treatme t of perforati g vei s, the CR40i catheter
ca be used. The catheter ca be i serted i the vei for
up to 25cm.
Thermocoagulatio of the perforati g vei s is performed
with a 0,5 cm lo g tip, which is pulled back slowly so the
e tire vei closes. Smooth a d fast i sertio is e sured by
the adva ced coati g material arou d the flexible core.
The marki gs allow for easy positio i g duri g pull-back.
4.3.9 CR45i catheter and 6 French Introducer
For the treatme t of the Saphe a Mag a a 6 Fre ch I troducer a d CR45i catheter are used.
The CR45i catheter is a special desig ed catheter for efficie t removal of the Saphe a Mag a.
Thermocoagulatio of the Saphe a Mag a is performed with a 0,5 cm lo g tip, which is pulled
back slowly so the e tire vei closes. Smooth a d fast i sertio is e sured by the adva ced
coati g material arou d the flexible core.
4.3.10 The 3M Pad
The eutral electrode is to be used whe worki g with a catheter type
CR45i. The eutral electrode is attached to the patie t.
The e tire area of eutral electrode/pad should be reliably attached to a
suitably prepared a d appropriate area of the patie ts’ body. We
recomme d the lower back, shoulder or buttocks area.
3M Pad
4.3.11 HPR45i probe
With the HPR45i probe hemorrhoids degree 2-4 an be treated easily and effe tively.
Other manuals for EVRF
2
Table of contents
Other F Care Systems Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual













