FingerTip LT-F20 User manual

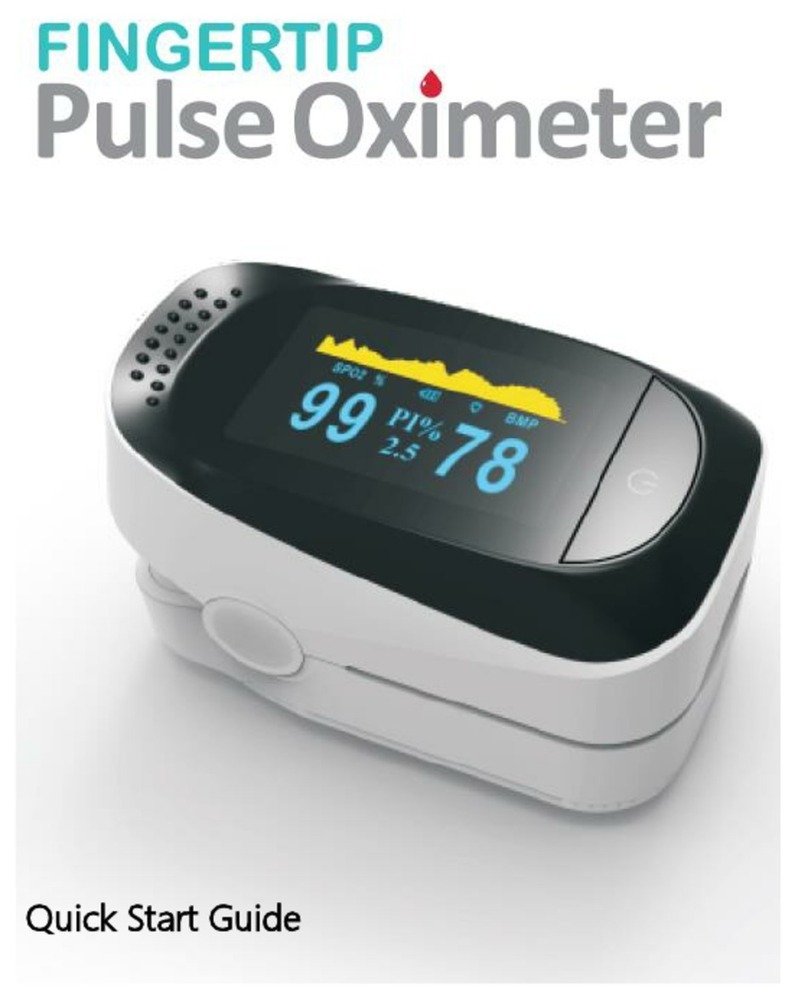
Fingertip Pulse Oximeter
User manual
(MODLE:LT-F20/LT-F21)
Welcome to use our product fingertip pulse oximeter. This manual introduces the product
performance, operation method and other safety information in detail. Please read this manual
carefully before using this device.

1
Chapter 1 Safety Guide
1.1 Electrical safety
Classified according to MDR: it is a Class IIa active (non-implanted) medical device.
Classified according to the type of protection against electric shock: equipment with internal
power supply.
Classified according to the degree of protection against electric shock: Type BF Applied Part.
Classified according to the degree of protection against harmful liquids: IP22.
Classified according to electromagnetic compatibility: Group 1 Class B.
1.2 Safety information
Warning: Information that you should know in order to avoid injury to patients and medical
staff.
Caution: Important information that should be emphasized.
1.2.1 Warning
1) Please do not use the product display information as the only basis for clinical diagnosis and
the product is only used as an auxiliary tool in diagnosis.
2) Before using the device, it should be under normal working condition and operating
environment.
3) The device should be used in a quiet and comfortable environment, not used during
exercise.
4) Ensure that the environment of the device is not disturbed by strong electromagnetic
interference sources, such as wireless transmitter, mobile phone, microwave oven, etc.
5) Do not disinfect pulse oximeter with high temperature, high pressure, gas fumigation or
liquid immersion.
6) This device is calibrated and maintained by professional technicians.
7) Keep equipment away from children, pets and insects.
8) The longest use of a single finger should not exceed 4h, otherwise it will lead to
overpressure injury.
9) Too much ambient light can affect measurement accuracy. Avoid using in strong sunlight and
dusty environment.

2
10) Do not use the instrument when the performance of the instrument changes.
11) Some patients may be allergic after prolonged exposure to the instrument. When allergy
occurs, please stop using the instrument immediately.
12) Use the battery recommended by the manufacturer. Use of other batteries may cause heat
or damage to the instrument.
13) No one except the manufacturer shall disassemble or modify the instrument.
14) The device and battery contains heavy metal and plastic which may do harm to environment,
please dispose according to local law.
15) LED light is a pollution of environment, it may harm to eyes for long time looking into LED.
16) MR UNSAFE: The device cannot be used in MRI environment.
17) The device shall not touch the damaged skin and wound.
1.3 Explanation of symbols
No.
Symbol
Explanation
1
BF type applied part
2
Refer to the manual
3
Caution, refer to the attached file
4
SPO2
Oxygen saturation
5
PR
Pulse rate
6
Disposal of waste electrical and electronic equipment
separately(Follow local government regulations and recycling
instructions for batteries)
7
No SpO2 Alarms
8
Serial number
9
IP22
Enclosure protection class
Protected against solid foreign objects of 12,5 mm ∅ and
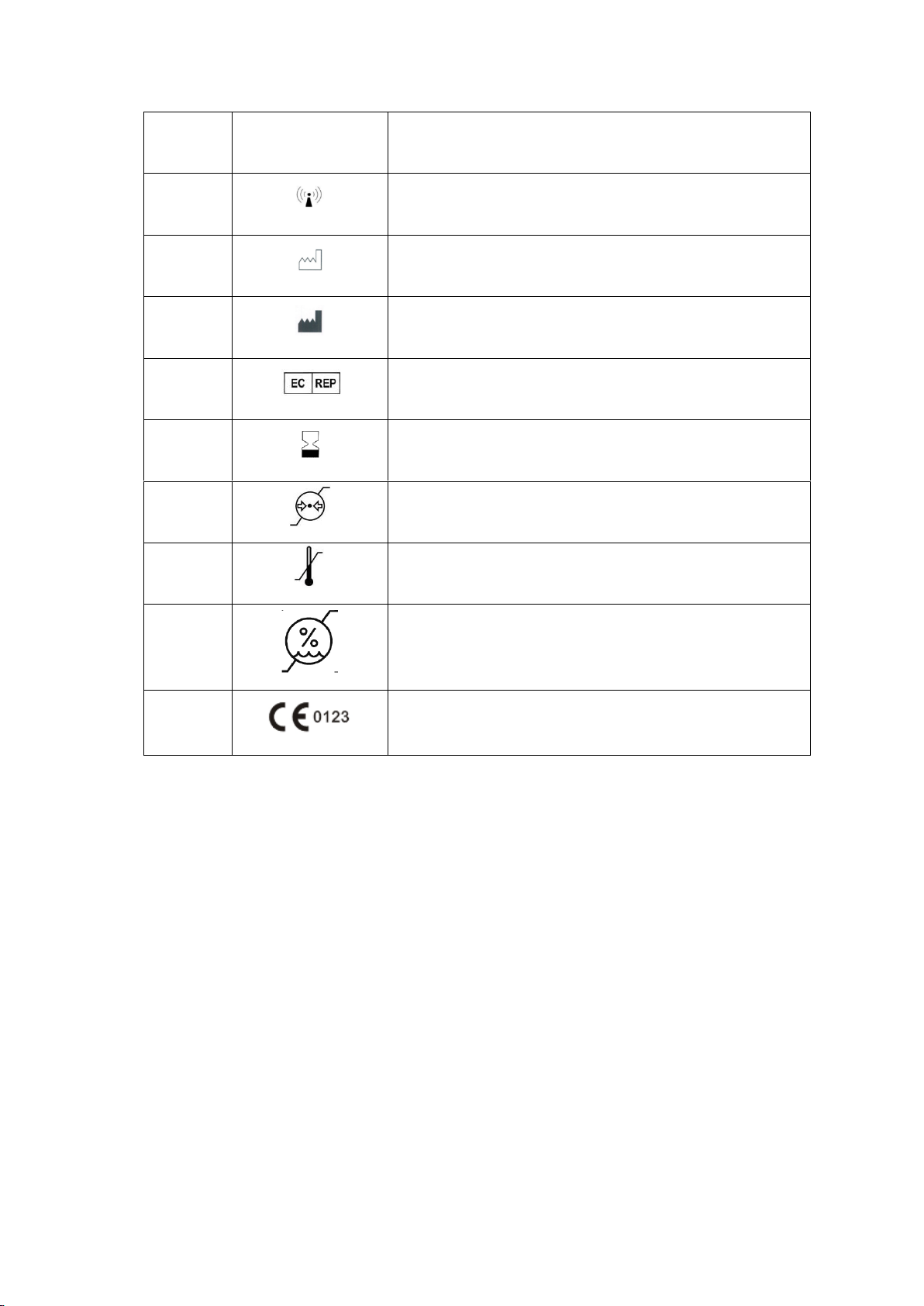
3
greater. And protected against vertically falling water drops
when enclosure tilted up to 15°
10
Non-ionizing radiation
11
Date of manufacture
12
Manufacturer
13
Authorized representative in the European Community
14
Use-by date
15
Atmospheric pressure limitation
16
Temperature limit
17
Humidity limitation
18
CE mark: indicates that the device complies with the EU
2017/745
NOTE: Any serious incident that has occurred in relation to the device should be reported to
the manufacturer and the competent authority of the Member State in which the user and/or
patient is established

4
Chapter 2 Product Overview
2.1 Model
LT-F20, LT-F21
2.2 Product Composition
It consists of shell, sensor, internal circuit and display screen.
2.3 Intended Use
The Fingertip Pulse Oximeter is intended for measuring functional oxygen saturation of arterial
hemoglobin (SpO2) and pulse rate for patients over 3 years old as non-invasive spot checking in
home and professional caring environment.
Contraindications: Do not use in patients with finger trauma.
The Fingertip Pulse Oximeter is forbidden for use in the patient with hypotension, severe
vasoconstriction, severe anemia or hypothermia and the patient in immediate danger.
2.4 Product Features
It can be used under the guidance of the doctor or by the patient himself, by holding it on the
finger to measure the blood oxygen saturation and pulse rate. It applies to adults and children.
Can be used by different patients, but can only be used by one patient at a time. No
contraindications.
2.4.1 The product is designed to be light in weight, simple in operation, easy to use and carry.
2.4.2 True Color high resolution OLED and LED.
2.4.3 It is only used for on-site monitoring and not used for continuous monitoring. It’s suitable
for hospitals, clinics and families.
2.5 Performance Parameters
Display Type Color high resolution OLED or LED display
Oxygen saturation Measurement range: 35%~100%
of blood(SPO2 ) Resolution: 1%
Accuracy: Within the range of 90% ~ 100%, tolerance ± 2%;
Within the range of 70% ~ 90%, tolerance ± 3%;
Less than 70% undefined
Pulse Rate(PR) Measurement range: No less than 30bpm ~ 250bpm(Real-time pulse
rate data after stabilization)
Resolution:1bpm
Accuracy: ±1% or ±3bpm, large value

5
Anti-ambient The deviation between the Spo2 measured in the room with day lighting
Light interference or illumination source and that measured in the darkroom is less than
Ability +1%
Power supply 2 x1. 5v ,AAA alkaline batteries(It can be used for 1 ~ 1.5 hours continuously)
Working current 30mA- 80mA
Direction sensor LT-F20: Digital and waveform display on the same screen, manual display in
four directions
LT-F21: Digital display, manual display in two directions.
Light sensor Red light wave length (657nm-663nm 7mW,Low power light in this wavelength
range is safe for the human body.)
Infrared light (wave length 900nm-910nm 55mW)
Data update cycle No more than 15s(From optical signal to digital signal)
2.6 Packing list
Description
Quantity
Main unit
1
Handing rope
1
2.7 Size and Weight
Length×Width×Height: 58.8×36×34mm
Weight: 54g (including batteries)
2.8 Environmental requirements
Working environment
Temperature : 5-40℃
Humidity : ≤80%(No condensation)
Atmospheric pressure :86-106 kPa
Storage and transportation condition
Temperature : -20~55 ℃

6
Humidity : ≤93%(No condensation)
Atmospheric pressure :70-106 kPa
Caution: The time from extreme storage environment to normal use of the instrument
should be no less than 3h.
2.9 Biocompatibility
The product is proved to be non-cytotoxic, non-allergenic and minimal skin irritation when
contacting with human body.
2.10 Conformity Declaration of Blood Oxygen
This product conforms to the standard requirements of ISO 80601-2-61.
2.11 Declaration of no alarm
This product does not have the alarm function and does not conform to the standard
requirements of IEC 60601-1-8.When abnormal measurements are found, consult a doctor or
visit the hospital in time.
2.12 The use time in the same position should not exceed 4 hours.
2.13 Statement of SPO2 measurement function
The verification of the accuracy of blood oxygen saturation was obtained by a clinical trial
comparison with the blood gas analyzer.
2.14 Demographic characteristics of clinical research
A) Age for male or female from 18 to 45;
B) No smoking history/ no history of tobacco addition;
C) No previous history of cardiopulmonary diseases;
D) The volunteers have the ability to act independently, agree to participate in the study, and sign
the informed consent;
E) The volunteers must be in a good state of mood when participating in clinical validation;
F) Blood pressure values of the volunteers: systolic blood pressure 90-140mmhg, diastolic blood
pressure 60-90mmhg;
G) Heart Rate of volunteers: 60-100 bpm;
H) The first arterial blood gas analysis of volunteers under breathing air:
SaO2 > 95%; COHb < 3%, MetHb < 2%, ctHb > 10g/dl;
I) The volunteers can have good compliance, and cooperate with the whole test;
J) The sample should meet the expected requirements of the clinical trial:
Subjects should include both male and female;

7
Adult volunteers should be able to withstand the minimal risk of an agreement for
controlled blood oxygen test requirement.
2.15 The incompleteness of blood oxygen signals
This product adopts normalized waveform, “---” is displayed to indicate signal inadequacy.
2.16 According to ISO 80601-2-61 standards, arterial blood oxygen saturation (SpO2) value
depends on the accuracy of the calibration curve of the pulse oximetry is properly reflected pulse
oximetry interact with pulse oximetry - the organization of the optical properties, functional
tester is unable to confirm the SpO2 accuracy calibration curve, also cannot fully evaluate the
optical properties of pulse oximetry to determine its validity, so the function tester cannot be
used to evaluate the accuracy of the pulse oximetry.
2.17 Product structure introduction (as figure 2.1)

8
Figure 2.1 product structure
Chapter 3 Battery Installation
3.1.Open the battery cover on the rear panel of the instrument.
3.2. Install two batteries of 1.5V AAA Alkaline correctly in the battery slot according to the
positive and negative polarity indication symbol.
3.Close the battery cover.(as figure 3.1)
Figure 3.1 close the battery over
Caution:
1. Replace the batteries when the batteries are insufficient.
2. Do not make short-circuit or positive and negative reverse loading when using batteries,
otherwise it may cause serious damage to the equipment.
3. If the pulse oximeter is not used for a long time, please remove the batteries from the battery
box, Otherwise, battery leakage may occur in the battery compartment.
4. After the battery is used, it must be disposed according to local regulations.
9
Chapter 4 Operation Method
4.1. Press the power button on the control Panel to open the pulse oximeter.
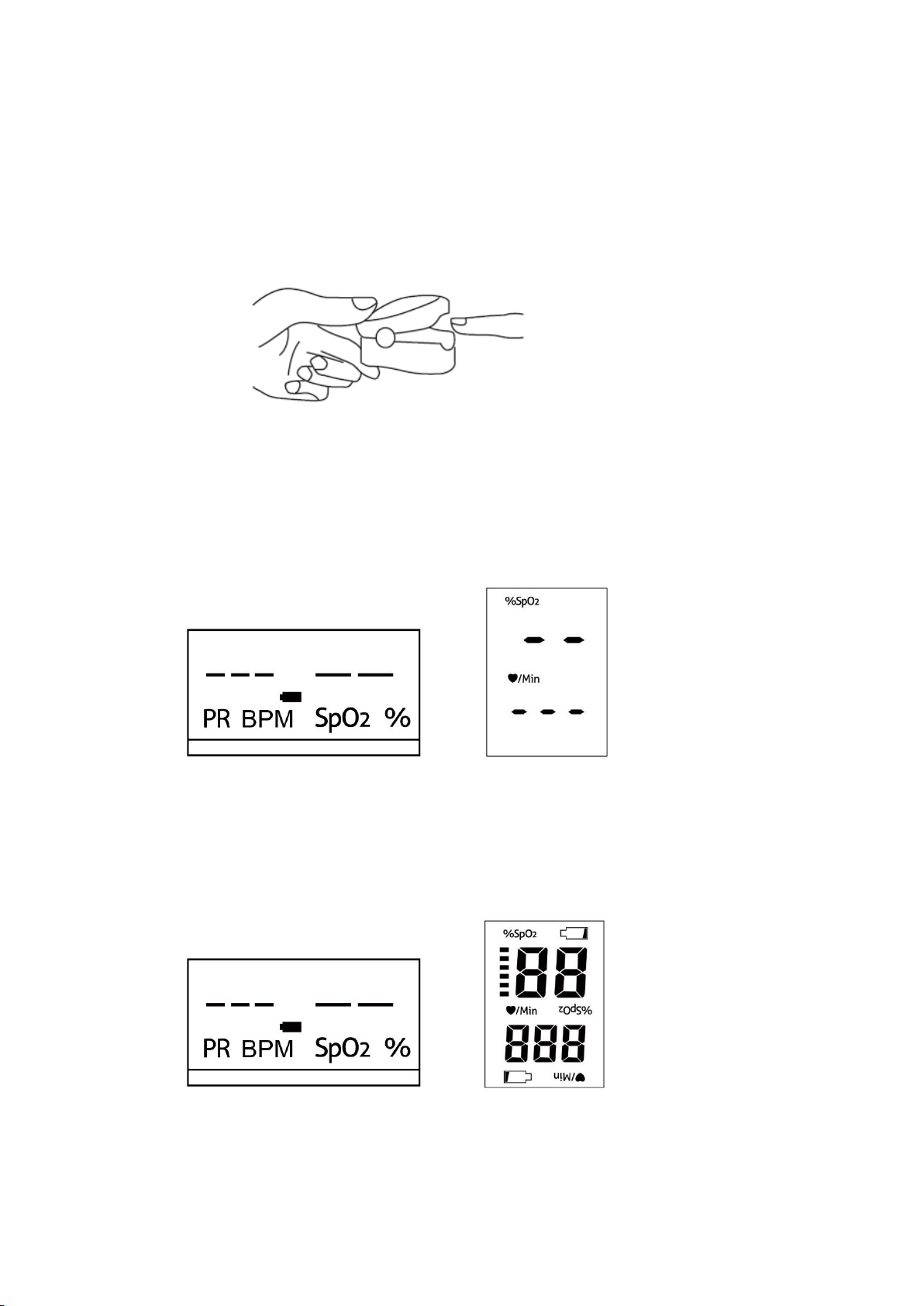
4.2. Insert the finger into the rubber finger sleeve. (as figure 4.1)
Figure 4.1 insert the finger
4.3.After the stable value (approximately 3-4s) is displayed on the screen, the monitor data is
displayed from the display, and the bar chart shows the pulse intensity. If the finger insert into
the fingertip pulse ,the screen only show the bar chart and pulse sound, it means the signal of
product is not sufficient to show the spO2 value and pulse value. (as figure 4.2 & 4.3)
Figure4.2 F20 unstable signal Figure4.3 F21 unstable signal
Normal usage test : when user insert the finger into the rubber finger sleeve, the screen
show normal as it show bar chart and pulse sound ,and SpO2 value ,pulse value after the
signal is stable.
Figure4.4 F20 screen interface Figure4.5 F21 screen interface
4.4.Screen display can rotate in two or four directions. Here are the options:

10
①Manual operation :When the pulse oximeter is turned on, press the power switch every time,
the display rotates 90 degrees.(suitable for LT-F20)
② Manual operation :When the pulse oximeter is turned on, press the power switch every
time, the display rotates 180 degrees.(suitable for LT-F21)
4.5.After reading the data, remove the finger, and the device will automatically turn off within 15
seconds.
Caution:
1. Ensure that the measured finger is free of lesions.
2. Hold fingers correctly and accurately in the direction indicated.
3. This pulse oximeter is neither suitable for continuous monitoring nor for the measurement of
newborns and infants.
4. Do not use this equipment in the presence of flammable anesthetics.
5. If the accuracy of equipment measurement is not determined, please first check the patient's
vital signs by an alternative method, and then check the oximeter. The inaccurate measurement
may be caused by the following factors:
① Ambient light interference.
② Body movement.
③ The influence of electromagnetic fields, such as the use of mobile phones nearby.
④Blood vessel stains or external coloring products, such as nail polish or color skincare products.
⑤Arterial blood is too low to measure, which is caused by shock, anemia, hypothermia or
vasoconstriction.
⑥Patients with severe smoking may appear instantaneously high in CO, resulting in hemoglobin
CO increased.
⑦Patients with severe jaundice will appear high bilirubin, the metabolism produced by CO will
produce important carboxyl hemoglobin, resulting in high SpO2.
⑧ The influence of electrical Surgery equipment.
6. For non-professionals, please read the instruction manual of the device carefully before using
it. If the device cannot provide valid data (as shown in Figure 4.2 and 4.3), please contact the
after-sales service personnel.

11
Chapter 5 Cleaning and Disinfection
The product housing and silicone liners can be cleaned and disinfected. Cleaning agent for water,
disinfectant for medical alcohol.
5.1 Cleaning
Please shut down and remove the battery before cleaning.
The instrument should be kept clean and free from dust or dirt. Use before and after usable a
clean, soft gauze dips in after taking clear water to undertake wiping.
5.2 Disinfection
If necessary, medical alcohol can be used for disinfection, before and after disinfection should be
used clean, soft gauze dipped in water to wipe clean.
To disinfect, wipe the entire surface of the device evenly with clean, soft gauze dipped in medical
alcohol for at least 3 times, and should be no less than 1 minute.
Caution:Do not use corrosive or abrasive cleaning agents.

12
Chapter 6 Maintenance and Troubleshooting
6.1 Maintenance
In order to ensure the service life of the machine, please pay attention to maintenance.
⚫Please replace the battery when the battery is low. If not in use for a long time, please take
out the battery.
⚫Necessary cleaning or disinfection should be carried out before and after use.
⚫Regular inspection to make sure that no obvious damage existed to affect the safety and
performance of device.
⚫No flammable substance, overtop or lower temperature and humidity existed in operation
conditions.
⚫It is better to preserve the product in a place where ambient temperature is -5 - 40℃ and
humidity is 15% - 85%.
⚫It is recommended that the equipment be placed in a dry environment. Damp environment
can affect the life of the product and damage it.
⚫Do not pour the liquid on the instrument.
⚫Please stop using the product and comply with local regulations and the recycling
instructions for disposal of waste when the device cannot operate because the battery is
exhausted, and when the light sensor is severely damaged, causing the device stop working.
⚫Do not dispose of electrical appliances as unsorted municipal waste, use separate collection
facilities. Contact the local government for information regarding the collection systems
available. Remove the battery and circuit board when discarding. Contact the local waste
disposal department to dispose of the battery and circuit board according to the specified
WEEE requirements. Dispose the shell materials as common garbage. If electrical appliances
are disposed of in landfills or dumps, hazardous substances can leak into the groundwater
and get into the food chain, damaging your health.
⚫calibration:The product is Calibration Not Required during its services life.
6.2 Troubleshooting
Problems
Possible causes
Solution
SPO2 or pulse rate
abnormally
display
Plug the finger correctly
Correctly put your finger and
retry

13
SPO2 or pulse rate
Are unstable
①Fingers may not be in the right
place.
②fingers tremble or people are in
motion.
① Retry to plug the finger
② Remain still
No boot
① battery power is too low or not
installed
②battery is not properly installed
③instrument may be damaged
① replacing the battery
② Make sure the battery is
installed
③ Contact Service
Representative
The screen
suddenly shut
down
①Instrument is shut down
automatically at 15s when the
finger signal is not detected for a
long time.
② The batteries are exhausted.
①Normal phenomenon
③Replace the new batteries
14
Chapter 7 Electromagnetic Compatibility Information
Electromagnetic Compatibility
Caution:
⚫The instrument conforms to the requirements of IEC 60601-1-2 standard for
electromagnetic compatibility.
⚫The users shall install and use according to the EMC information provided in random file.
⚫Portable and mobile RF communication equipment may affect the performance of the
instrument, avoid strong electromagnetic interference when using, such as close to the
mobile phone, microwave oven, etc.
⚫The guidance and manufacturer's declaration are detailed in the annex.
Warning:
⚫The instrument should not be close to or stacked with other equipment. If it must to be
close to or stacked, it should be observed and verified to be able to operate normally under
its configuration.
⚫In addition to the cables sold by the instrument manufacturer as spare parts for internal
components, the use of other accessories and cables may result in increased emission or
reduced immunity.
Guidelines and manufacturer's statement - electromagnetic emission
The instrument is intended to be used in the electromagnetic environment specified below,
and the purchaser or user of the instrument should guarantee that it is used in this
electromagnetic environment:
Emission test
conformity
Electromagnetic environment –guidelines
RF emission
EN 55011
Group 1
The instrument uses RF power only for its
internal functions. Therefore, its RF emission
is low and the possibility of interference to
nearby electronic devices is minimal.
RF emission
EN 55011
Class B
The instrument is suitable for use in all
facilities, including household facilities and
public low-voltage power supply networks
directly connected to household homes.
Harmonic emission
IEC 61000-3-2
N/A
15
Voltage fluctuation/flicker
emission
IEC 61000-3-3
N/A
Guidelines and manufacturer's statement – Electromagnetic immunity
The instrument is intended to be used in the electromagnetic environment specified below, and
the purchaser or user of the instrument should guarantee that it is used in this electromagnetic
environment:
Immunity test
IEC 60601 test levels
Applicability levels
electroma
gnetic
environme
nt
-
guidelines
electrostatic discharge
IEC 61000-4-2
±8 KV contact discharge
±2kV, ±4kV, ±8kV,±
15kV Air discharge
±8 kV Contact
discharge
±2kV, ±4kV, ±
8kV, ±15 kV Air
discharge
The
ground
should be
wood,
concrete
or ceramic
tile, if the
ground is
covered
with
synthetic
materials,
the
relative
humidity
should be
at least
30%.
Electrical fast
transients/bursts
IEC 61000-4-4
±2kV
100KHz repetition
frequency
N/A
/
Surges
IEC 61000-4-5
±1 kV line to line
±2 kV lines to ground
N/A
/
Voltage dips
IEC 61000-4-11
0%UT;0.5 cycle
At 0°,45°,90°,
135°,180°,225°,
270°and 315°
0%UT;1 cycle and
N/A
/

16
70%UT;25/30 cycles
Single phase: at 0°
Voltage interruptions
IEC 61000-4-11
0%UT;250/300 cycle
N/A
/
RATED power frequency
magnetic fields
IEC 61000-4-8
30A/m
30A/m,50/60Hz
/
Note: UT refers to the voltage of the AC network before the test voltage is applied.
Guidelines and manufacturer's statement – Electromagnetic immunity
The instrument is intended to be used in the electromagnetic environment specified below,and
the purchaser or user of the instrument should guarantee that it is used in this electromagnetic
environment:
Immunity test
IEC 60601 test level
Applicability level
electromagnetic
environment-guideli
nes

17
Conducted disturbances
induced by RF fields
IEC 61000-4-6
Radiated RF EM fields
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
6 Vrms
150 kHz to 80 MHz
80% AM at 1 kHz
10V/m
80 MHz to 2.7 GHz
80% AM at 1 kHz
N/A
N/A
10V/m
Portable and mobile
RF communications
equipment should
not be used closer to
any part of the
instrument than
recommended
isolation distances,
including cables. The
distance shall be
calculated by a
formula
corresponding to the
frequency of the
transmitter.
Recommended
isolation distance
d =
P2.1
d =
P2.1
80
MHz~800 MHz
d =
P3.2
800
MHz~2.7 GHz
In the formula:
P
—
According to the
maximum rated
output of
transmitters
provided by
transmitter
manufacturers, Watt
(W) is the unit;
d—The
recommended
isolation distance,
Meter(m) is the unit.
18
The field strength of
a stationary RF
transmitter is
determined by
surveying (c) the
electromagnetic
site,and (d) should
be lower than the
applicability level in
each frequency
range .
Interference may
occur near the
device that marks
the following symbol
19
Note1:In the frequency of 80MHz and 800MHz, the higher frequency formula is adopted.
Note2:These guidelines may not be suitable for all situations, where electromagnetic transmission
is affected by the absorption and reflection of buildings, objects and bodies.
a. Stationary transmitter,such as base stations for wireless (cellular/cordless) telephones and
ground-based mobile radios, amateur radios, AM and FM radio broadcasts and television
broadcasting, are theoretically predicted accurately in the field strength. In order to evaluate the
electromagnetic environment of the Stationary RF transmitter, the investigation of the
electromagnetic field should be considered. If the measured field strength is higher than the
applicable RF conformance level, the instrument should be observed to verify its normal operation.
If abnormal performance is observed, supplementary measures may be necessary, such as such as
re-adjusting the orientation or position of the instrument.
b. In the whole frequency range from 150KHz to 80MHz, the field strength should be less than 3
V/m.
Recommended isolation distance between portable and mobile RF communication devices and
instruments
The instrument is expected to be used in the electromagnetic environment controlled by radio
frequency radiation disturbance. Depending on the maximum rated output power of the
communication equipment, the purchaser or user can prevent electromagnetic interference by
maintaining the minimum distance between the portable and mobile radio frequency communication
equipment (transmitter) and the instrument as recommended below.
Rated maximum output
power of the transmitter
/w
Isolation distance for different frequencies of the transmitter/m
150 kHz ~ 80 MHz
d =
P2.1
80 MHz ~ 800 MHz
d =
P2.1
800 MHz~ 2.7 GHz
d =
P3.2
0.01
N/A
0.12
0.23
0.1
N/A
0.38
0.73
1
N/A
1.2
2.3
10
N/A
3.8
7.3
100
N/A
12
23
This manual suits for next models
1
Table of contents
Other FingerTip Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Meditrac
Meditrac Vertetrac and DBS user manual
SKEEPER
SKEEPER Mozart Manual instruction

Saviour Medical
Saviour Medical Aquatic Sled user manual

Mangar International
Mangar International Leglifter User instructions and warranty

Blatchford
Blatchford S400 Instructions for use
Boston
Boston SpyGlass User reference guide