2
1. INTRODUCTION .......................................................................................................................................... 4
Programming modes .................................................................................................................................... 4
Infusion modes ............................................................................................................................................. 4
Intended use ................................................................................................................................................. 5
Precautions to be taken ................................................................................................................................ 5
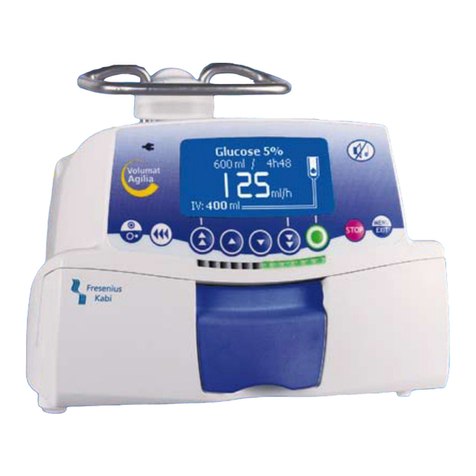
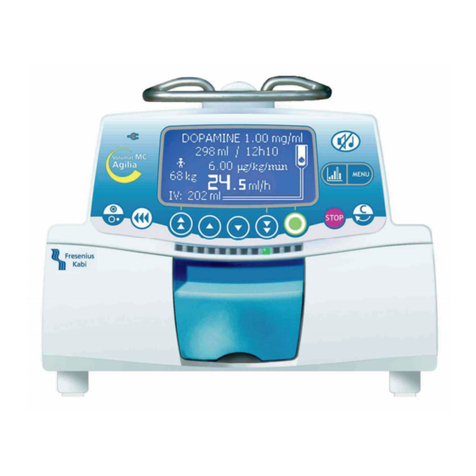
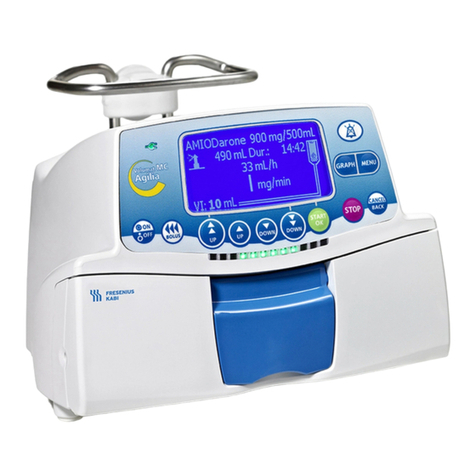
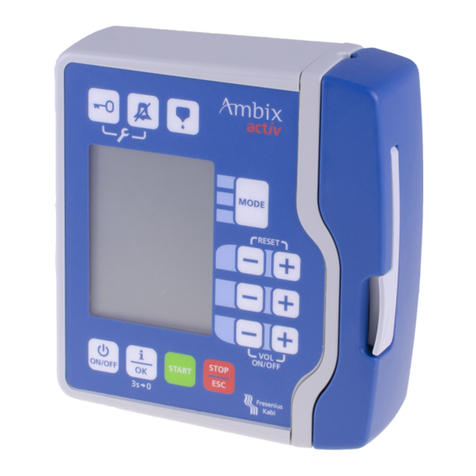
2. DESCRIPTION ............................................................................................................................................. 6
3. INSTALLATION ........................................................................................................................................... 8
4. OPERATIONS ............................................................................................................................................ 13
No drug name and flow rate ml/h modes.................................................................................................... 13
Other infusion modes in flow rate ml/h ....................................................................................................... 14
No drug name and dose rate mode ............................................................................................................ 20
Loading dose .............................................................................................................................................. 22
Other infusion modes in dose rate.............................................................................................................. 24
Drug labelling mode.................................................................................................................................... 25
Vigilant Drug’Lib mode................................................................................................................................ 26
Special features .......................................................................................................................................... 27
General operations ..................................................................................................................................... 30
History......................................................................................................................................................... 32
5. DISPLAY AND SYMBOLS......................................................................................................................... 36
6. ALARMS AND SAFETY FEATURES ........................................................................................................ 39
7. MENU ......................................................................................................................................................... 42
Permanent menu ........................................................................................................................................ 42
Menu selected in option mode.................................................................................................................... 43
8. OPTIONS.................................................................................................................................................... 45
9. USER TEST................................................................................................................................................ 50
10. PERFORMANCE........................................................................................................................................ 51
Rates range ................................................................................................................................................ 51
Volume to be infused (VTBI)....................................................................................................................... 51
KVO (Keep Vein Open) rate ....................................................................................................................... 51
Dose range ................................................................................................................................................ 52
Infusion time ............................................................................................................................................... 52
Drug library ................................................................................................................................................. 52
Air detection................................................................................................................................................ 52
Set replacement interval ............................................................................................................................. 52
Accuracy ..................................................................................................................................................... 53
Programmable pause ................................................................................................................................. 53
Pressure management .............................................................................................................................. 53
Occlusion alarm response time .................................................................................................................. 53
Bolus volume at occlusion release ............................................................................................................. 54
Calculation rules ......................................................................................................................................... 54
Units and conversion rules ......................................................................................................................... 55
Contents