User Manual
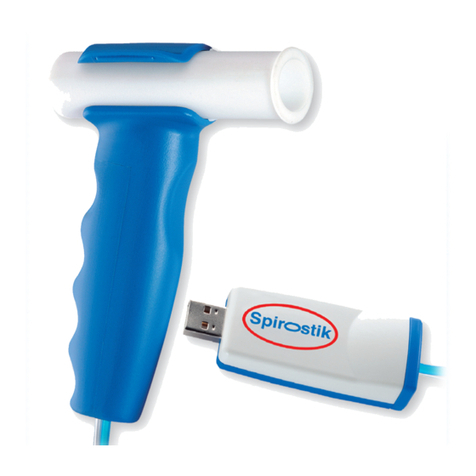
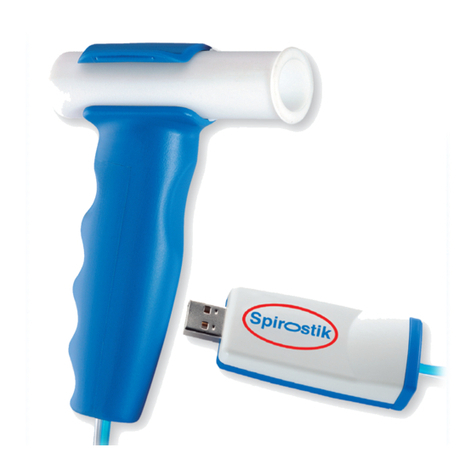
Ergostik
ERGOSTIK_MAN_ENG_V1.2.2_REV01.DOCX
1. Welcome to Blue Cherry
Thank you for choosing the Blue Cherry diagnostic platform, we are sure you will be very happy
with your choice, and find the advanced functionality offered by Blue Cherry a great benefit in
your diagnostic activities.
The Blue Cherry diagnostic platform offers its user a level of functionality which is unsurpassed,
providing multiple diagnostic measurements from within a single software platform. Addition
of further diagnostic measurements couldn’t be easier: simply purchase the required hardware
and plug it into a spare USB 2.0 port on the PC. The Blue Cherry system will recognise the new
device and testing will become available within the diagnostic platform.
The Blue Cherry platform works well as a standalone device for the smallest of requirements,
however expansion for the future is simple. The software has been designed to allow
connection of multiple units to a single Blue Cherry Database to allow centralisation of data, for
the purposes of reporting or statistical analysis, further with the ability to interconnect with
many Hospital information systems using the HL7 format, the diagnostic system can be
integrated into even the largest of requirements.
This document contains important information for the operation of Blue Cherry and Ergostik.
We strongly recommend reading this manual carefully in order to avoid incorrect use or
damage to the device. Geratherm Respiratory does not take responsibility for any direct or
indirect damage to the Device, if it is not operated in accordance with this manual. Users must
observe precautions, warnings and instructions.
Geratherm Respiratory does not take liability for mistakes in this documentation. The liability
for direct or indirect losses related to the use of this document is excluded, as long as this is
allowed by law.
The copyright for Blue Cherry is owned by Geratherm Respiratory. Permission is given to use
the Blue Cherry software provided on the installation CD upon acceptance of the EULA. (see
appendix) Blue Cherry™ and Spirostik™ remain trademarks of Geratherm Respiratory.
This user manual is according to EN 60601-1 an integral part of the product. Geratherm
Respiratory reserves the right to make changes to the contents without prior notice. All
changes made are in accordance with the guidelines for manufacturing medical devices.
Geratherm Respiratory GmbH
Sparkassenpassage 1 info@geratherm-respiratory.com
D-97688 Bad Kissingen www.geratherm-respiratory.com
Tel. +49 (0)971 7857043-0 Fax +49 (0)971 7857043-30
© Copyright 2007 Geratherm Respiratory GmbH