Infinite Element Assembly instructions
1
Element
Installation and Setup Guide
for the Prosthetist

2 3
Thank you for choosing Element to provide EMG inputs for an upper limb
myoelectric prosthesis. This guide will familiarize you with Element and help
you install the hardware and software.
Have any questions? We’re happy to help. Call us or send us an email.
(443) 451-7175
INTENDED USE
The Element system is intended to detect, process, and transmit physiological
signals for use with a prosthesis
INDICATIONS FOR USE
The Element system is intended to be used exclusively for myoelectric exo-
prosthetic fittings of the upper limb.
CONDITIONS FOR USE / PATIENT TARGET GROUP
Element is intended for use on one patient only, for users with unliteral or
bilateral amputation, hand, forearm and upper arm amputation or dysmelia.
Use of the product by another person is not approved by the manufacturer.
Installation of the system should be performed exclusively by a licensed
prosthetist or technician. Any unauthorized handling or installation of Element
could void its warranty.
All rights reserved. Element is a trademark of Infinite Biomedical
Technologies, LLC.
This document provides information for the prosthetist who will be installing
Element and IBT Electrodes.
Element
INSTALLATION AND SETUP GUIDE
FOR THE PROSTHETIST
This symbol is used throughout the guide to indicate important
cautionary information. Text following this symbol should be
read carefully.
Caution: Federal law restricts this device to sale by or on the
order of a prosthestist.
This device includes an RF transmitter or applies radio frequency
electromagnetic energy.
Mdi Europa GmbH
Langenhagener Str. 71
30855 Langenhagen
Germany
SRN: DE-AR-000006218
LEGEND OF SYMBOLS USED
Medical
Device
Consult
Instructions
for Use
Single
Patient,
Multiple
Use
ImporterDistributorKeep
Dry
Translated
Serial
Number
ManufacturerEuropean
Authorized
Representative
CE Mark
Catalogue
Number
Model
Number
Contains FCC ID: XDULE40-D2 Contains IC: 8456A-LE4D2
Infinite Biomedical Technologies, LLC.
8 Market Place, Suite 500
Baltimore, MD 21202
(443) 451-7175
www.i-biomed.com
SRN: US-MF-000007619 www.i-biomed.com/support.html

4 5
Table of Contents
1 Meet Element ����������������������������������������������� 5
2 Component Description���������������������������������������� 7
3 Specifications ����������������������������������������������� 8
4 Installation ������������������������������������������������� 9
Before you Begin ������������������������������������������� 9
Connecting IBT Electrodes and Batteries ����������������������� 10
Powering Element with FlexCell ������������������������������ 11
Element Software ����������������������������������������� 12
Incorporating IBT Electrodes Into Socket������������������������ 17
Installing Element into the Prosthesis �������������������������� 21
5 Testing Element��������������������������������������������� 26
Troubleshooting������������������������������������������� 26
6 Maintaining Element ����������������������������������������� 26
Maintenance ��������������������������������������������� 26
Disposal������������������������������������������������� 27
Repairs, Returns, and Warranty������������������������������� 27
7 Safety and Warnings ����������������������������������������� 28
8 Regulatory Info ��������������������������������������������� 29
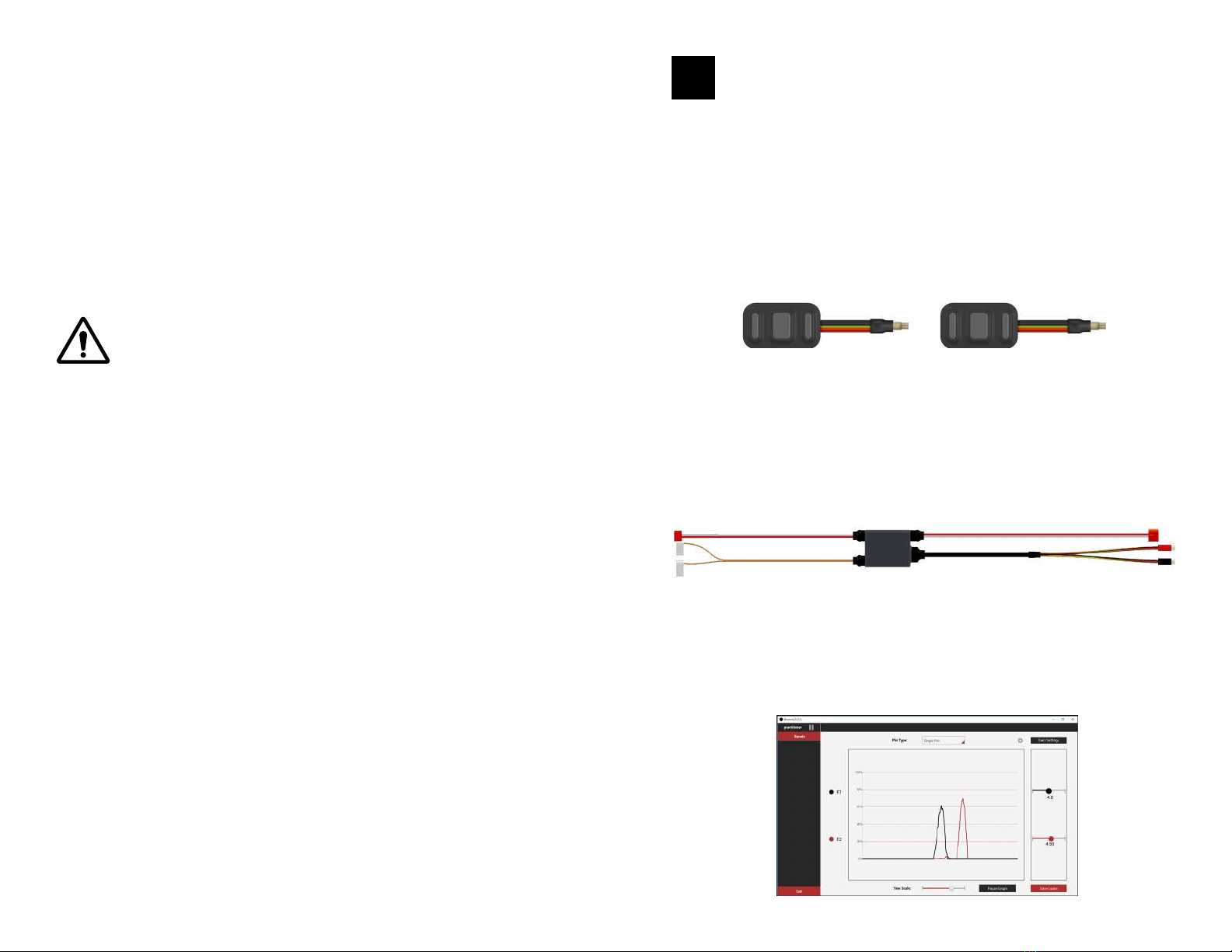
The Element System has three components:
• IBT Electrodes (up to 2)
• Signal Processing Box
• Element Desktop Software
Element should only be powered by FlexCell Batteries.
Element outputs standard envelope EMG signals detected from the IBT
Electrodes placed on the user’s skin. The Element System is an alternative to
standard suction socket myoelectrodes, with the advantages of a lower profile,
digital signal processing, and wireless gain adjustment. Element does not
replace or modify any functionality of connected prosthetic components.
Meet Element
1
6 7
Element is compatible with most hands, wrists, and elbows that accept standard
analog EMG electrode inputs. The Element system is typically sold with three-
port kidney-style connectors to connect with quick-disconnect terminal devices.
Alternative connectors may be available upon request. If you have a question
about compatibility with other devices, please contact us.
For detailed information on connections and cables, refer to the Element
Connections Guide.
The Element system was developed for everyday use and must not
be used for unusual activities. These unusual activities include, for
example, sports with excessive strain and/or shocks to the wrist
unit (pushups, downhill mountain biking) or extreme sports (free
climbing, paragliding, etc.). Furthermore the Element system should
not be used for the operation of motor vehicles, heavy equipment
(e.g. construction machines), industrial machines or motor-driven
equipment.
IBT ELECTRODES
SIGNAL PROCESSING BOX
ELEMENT SOFTWARE
The electrodes detect and amplify raw electromyography (EMG) signals from
the user’s skin. The electrodes plug into the signal processing box.
The signal processing box collects and filters the electrode EMG signals, and
outputs envelope EMG signals to the terminal device. The signal processing box
contains a Bluetooth module, which allows Element to communicate with the
desktop software.
The user can visualize EMG signals and adjust electrode gains through the
desktop software.
Component
Description
2

8 9
Dimensions (Element Box LxWxH) 38mm x 22.8mm x 3.85mm
Dimensions (IBT Electrodes LxWxH) 28.8mm x 16.8mm x 6.7mm
Temperature range (use) -10°C to +50°C (14°F to 122°F)
Temperature range
(transport/storage)
-20°C to +65°C (-4°F to 149°F)
Humidity range (use) 45% - 75%
Humidity range
(transport/storage)
15% - 93%
Atmospheric pressure range 860 hPa - 1060 hPa
Input voltage 5 to 10V
Maximum Output Current 3A
Compatible battery FlexCell
Recommended battery capacity Depends on terminal device. Contact
us for recommendations.
Expected service life 3 years
Compatible electrode IBT Electrodes
Bluetooth FCC, IC, CE, RoHS, and Bluetooth®
4.0 Certified ISM 2.4GHz module
Hands Wrists Elbows
SensorHand Speed bebionic small
MC ProWrist (with 4 or
6 band coaxial plug)
DynamicArm
MyoHand VariPlus Speed i-limb access
ProHand i-limb ultra
ProETD i-limb ultra revolution
Ottobock OB 10S17 with
Myorotronic
Steeper MyoHand i-limb quantum
bebionic3
Voltage Output 7.4V DC
Capacity Range * 550 mAh - 2200 mAh
Current Output Up to 7A
Temperature range (use) 0oC to +49oC (32oF to 120oF)
Temperature range
(transport and storage)
0oC to +49oC (32oF to 120oF)
Included in the Package
• Element signal processing box
• IBT Electrodes
• Molding dummies for IBT Electrodes
• Molding dummy for signal processing box (if requested)
• USB Thumb drive containing Element desktop software
• Bluetooth Adapter
• FlexCell batteries (if ordered with Element)
What You’ll Need
• PC
• FlexCell batteries (if not ordered with Element)
• Terminal device
• Coaxial plug (if not using a wrist or elbow)
• Lamination collar parts
Terminal Devices That Have Been Tested For Compatibility With Element
For FlexCell
* Capacity range is dependent on how many FlexCell batteries are installed.
BEFORE YOU BEGIN
Specifications Installation
3 4
Table of contents
Other Infinite Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual













