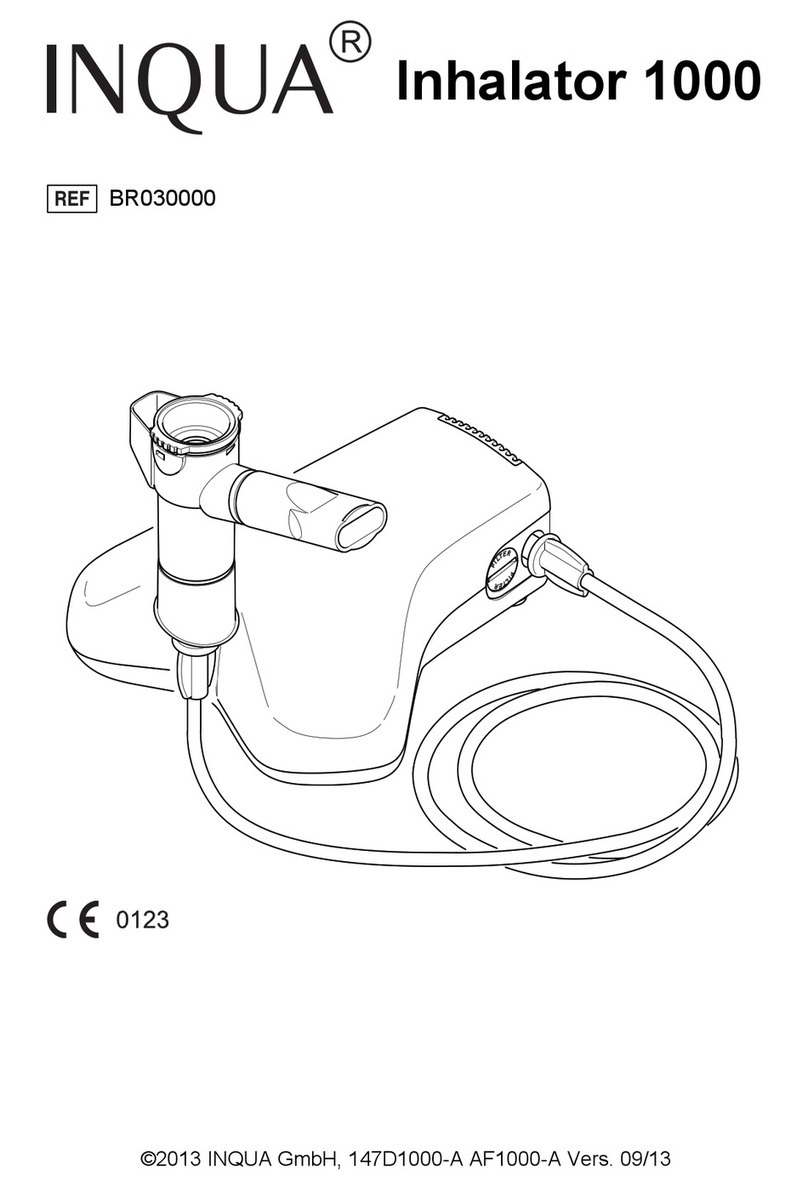
INQUA Profi BR021100 User manual

INQUA®Profi Vernebler
©2009,AE1000CVers.01/09


en
21
INQUA®Profi nebuliser
Instructions for use
TABLE OF CONTENTS
GENERAL_________________________________________________ 22
Manufacturer's data 22
Notes on the instructions for use 22
PRODUCT DESCRIPTION ____________________________________ 23
Components 23
Special notes 24
Correct use 25
Conformity 25
SAFETY INSTRUCTIONS ____________________________________ 26
Representation of hazard warnings 26
Product safety 26
APPLICATION _____________________________________________ 27
Preparing for inhalation 27
Performing the inhalation 29
Completing the inhalation 31
HYGIENIC RE-USE _________________________________________ 32
Preparation for cleaning/disinfection 33
Cleaning with warm tap water 34
Disinfection 34
Drying 35
Storage 35
Care of the connection tubing 36
MISCELLANEOUS __________________________________________ 37
Storage and transportation conditions 37
Material resistance 37
Disposal 37
SPARE PARTS/ACCESSORIES _______________________________ 38
TECHNICAL DATA _________________________________________ 38
General 38
Aerosol characteristics in accordance with EN 13544-1 appendix CC 39

22
GENERAL
Manufacturer's data
See back of instructions for use.
Notes on the instructions for use
Purpose of the instructions for use
These instructions for use describe how the INQUA®Profi nebuliser is as-
sembled, how it works, and how it should be used. They are intended to en-
sure that the individual components can be used in complete safety.
Instructions for using the compressor that will be attached to the nebuliser are
not included in these instructions for use. Please read the instructions for use
of the compressor which accompany that item.
Forms of emphasis
The following forms of emphasis are used in these instructions for use to in-
dicate important parts of the text:
àHazard warnings are indicated by a warning triangle and a signal word
(see also "Representation of hazard warnings", page 26).
àÖThe arrow indicates that a given action must be taken.
Read these instructions carefully and completely before using for the
first time. Do not use the INQUA
®
Profi nebuliser unless you fully un-
derstand the contents of these instructions for use.

en
23
PRODUCT DESCRIPTION
Components
1Nebuliser upper section 6Child mask
2Mouthpiece 7Air filter for the compressor
manufactured by INQUA®
3Nebuliser lower section
4Connection tubing 8Removal tool for changing the
filter
5Adult mask
1
3
4
front
5
6
2
8
7

24
Special notes
Note that the nebuliser upper
section (1) and the nebuliser
lower section (3) are attached
to each other by a "bayonet"
closure.
ÖThe nebuliser upper sec-
tion is inserted carefully into
the nebuliser lower section.
ÖPay particular attention to
the indication front in the
drawing.
The lugs in the nebuliser
upper section are different
sizes and only fit in the dif-
ferent sized slots in the
nebuliser lower section in a
certain direction.
Twist the nebuliser upper section clockwise while pressing gently to engage
the lugs on the nebuliser upper section in the slots in the lower section. The
two nebuliser sections will be attached securely by gently twisting clockwise.
front
Lug
Slots for
the lugs
1
3

en
25
Correct use
Together with the compressor being used, the INQUA®Profi nebuliser forms
a highly effective inhalation device for treatment of the upper and lower res-
piratory tracts with nebulised medication. It is suitable for temporary1) oral or
nasal inhalation of liquid medication prescribed or recommended by a doctor.
The INQUA®Profi nebuliser must only be used for inhalation treatment. Any
application other than the use described here is considered improper, and
therefore dangerous. In all cases the user is liable for the safe operation of
the nebuliser if it is not used according to the instructions.
The INQUA®Profi nebuliser is intended exclusively for use in the home and
together with the INQUA®compressor.
Conformity
The INQUA®Profi nebuliser satisfies the requirements of Directive
93/42/EEC (EC Medical Device Directive).
1. Each application less than 1 hour according to European Medical Device
Directive 93/42/EEC, in this case max. 20 minutes.

26
SAFETY INSTRUCTIONS
Representation of hazard warnings
In these instructions for use, hazard warnings are indicated by a warning tri-
angle and a signal word. The following signal words are used depending on
the nature and degree of danger being described.
àCAUTION
Indicates a hazard that may cause mild to moderate injury or damage if it
is not avoided.
àWARNING
Indicates a hazard that may cause serious injury or death if it is not
avoided.
Product safety
àINQUA GmbH cannot be held responsible for damage that is caused by
improper or incorrect use, e.g., use of a compressor made by another
manufacturer. Therefore, you are advised to read the instructions for use
of the INQUA®Profi nebuliser and the INQUA®compressor carefully and
to familiarise yourself with the device. It is particularly important to fol-
low the instructions for use of the INQUA®compressor.
àOnly use medication prescribed or recommended by your doctor.
àYou must conscientiously follow the general hygiene procedures when
using your nebuliser, e.g., thorough washing of hands or disinfection if
required.
àUse only liquid medications that have been approved for use in inhalation
therapy with a compressed air inhalation device in your INQUA®Profi
nebuliser. Ask your doctor or pharmacist about the selection and applica-
tion of the medications available.
àIn general, the following are not suitable for use in inhalation devices:
cough mixtures, gargles, balsamic preparations, oils of medicinal plants,
and drops that are used as ointments or added to the bath. Inhaling these
medications can cause irreversible damage to the respiratory tracts.
Such substances can also impair the function of the inhalation device
permanently.

en
27
àPlease use only accessories and spare parts that are recommended by
INQUA in order to obtain the best possible treatment (see "Spare parts/
Accessories", page 38).
àIt is most important to follow the instructions in chapter "Hygienic re-use",
page 32.
APPLICATION
Preparing for inhalation
Filling with inhalation solution
WARNING: Danger of infection from contaminated nebuliser
Follow the hygiene regulations each time when preparing the system
for use and after any relatively long interruption in use. Make sure
that the nebuliser has been cleaned and disinfected since the last
treatment (see "Hygienic re-use", page 32).
ÖPour the quantity of inhalation solution pre-
scribed by your doctor or the manufacturer into
the top of the nebuliser.
Make sure that the liquid added does not pass
the top scale graduation mark (max. fill quanti-
ty 8 ml).
If you overfill the nebuliser and medication
drips out of the bottom of the nebuliser, pour all
the liquid out and clean the INQUA®Profi neb-
uliser (see Section "Cleaning with warm tap
water", page 34).
ÖDo not fill with medication again until the
INQUA®Profi nebuliser has been cleaned
thoroughly.
max.

28
Assembling the nebuliser
ÖAttach the mouthpiece (2) or the
respective mask (5) or (6) to the
nebuliser.
ÖConnect one end of the connec-
tion tubing (4) to the air outlet
(AIR) on the INQUA®compressor
(see the instructions for use of the
INQUA®compressor) and the
other end to the INQUA®Profi
nebuliser.
CAUTION: Inadequate medication nebulising due to incorrect
assembly
Before inhaling, ensure that all parts are firmly connected to each
other. Make sure that the tubing connections are fitted securely to
the air outlet (AIR) on the INQUA®compressor and to the nebuliser.
2
5
4
6

en
29
Performing the inhalation
Inhalation for the lower respiratory tract
Inhalation with the mouthpiece is the most effective form of inhalation for the
lower respiratory tract, since it results in minimum medication loss on the way
to the lung. Children's and adult masks are only recommended for patients
who are unable to inhale with a mouthpiece.
WARNING: Danger to health from improper use
Contains small parts which can be swallowed!
Please note that babies, infants and anyone who requires assistance
must be supervised constantly during the inhalation treatment.
CAUTION: Inadequate delivery of medication because nebuliser
held incorrectly
Always hold the nebuliser upright during inhalation.
ÖSit in an upright position and relax.
ÖSwitch the compressor on.
The nebuliser will generate a visible mist
(aerosol).
ÖHold the mouthpiece between your teeth and
enclose it with your lips.

30
ÖBreathe in as gently as possible through the
mouthpiece.
ÖAfter a few seconds, breathe out through your
nose.

en
31
Inhalation for the upper respiratory tract
ÖHold the nebuliser vertically, sit in a relaxed, upright position.
ÖSwitch the compressor on.
The nebuliser will generate a visible mist (aerosol).
Completing the inhalation
The duration of inhalation depends on the quantity of medication used and
the breathing pattern.
Inhalation is complete when no more aerosol is produced by the nebuliser, or
the aerosol only emerges from the nebuliser intermittently.
ÖWhen the inhalation session has ended, turn the INQUA®compressor off
at the On/Off switch.
ÖPull the power plug of the power cord out of the socket (see instructions
for use of the INQUA®compressor) and disconnect the connection tubing
from the nebuliser.
ÖHold the mask up to your face so that it
forms an airtight seal over your mouth
and nose.
ÖInhale slowly and evenly through your
nose, and after a few seconds exhale
through your nose again.
WARNING: Bacterial contamination caused by residues
Bacteria can breed very quickly in nebulisers that have not been
cleaned properly or liquids that are left in the nebuliser, thereby
endangering health, for example by causing infections.
You should therefore begin preparation for hygienic re-use as soon
as the inhalation session is ended. Always empty out the residue in
the nebuliser and never store any liquids in it.

32
HYGIENIC RE-USE
The INQUA®Profi nebuliser is designed for multiple uses. It must not be used
by more than one person. A separate nebuliser must be provided for each pa-
tient.
To do this, follow these instructions:
àThe nebuliser must be cleaned and disinfected after each use.
àAdditional requirements concerning the necessary hygienic preparations
(hand disinfection, handling of medications and inhalation solutions) in
high-risk groups (e.g., users with suppressed immune systems, patients
with cystic fibrosis) can be obtained from the respective self-help groups.
àPlease ensure that the INQUA®Profi nebuliser is dried thoroughly after
each cleaning and disinfection. Condensation or residual wetness can
present an increased risk through the growth of bacteria.
àPlease follow the instructions in Chapter "Material resistance", page 37.
àCheck the parts of your nebuliser regularly and replace any defective (bro-
ken, misshapen, discoloured) parts (see "Spare parts/Accessories",
page 38).
àPlease also read and follow the hygiene instructions in the instructions for
use of the INQUA®compressor.
Like all plastic parts, the INQUA®Profi nebuliser is susceptible to a certain
degree of wear when it is used and reprocessed frequently. Over time, this
can cause a change in the aerosol, which in turn impairs the therapeutic
efficiency.
You should replace the nebuliser at least once a year. Replacement sets can
be obtained from your sales partner or from INQUA GmbH (see "Spare parts/
Accessories", page 38).
Proof of suitability of the INQUA®Profi nebuliser for effective cleaning and
disinfection has been obtained through an independent testing laboratory us-
ing the procedures recommended on page 34. Using the named alternatives
remains the responsibility of the user.
WARNING:
In order to prevent a risk to health, e.g., infection due to a contami-
nated nebuliser, it is imperative that the following hygiene regulations
are followed.

en
33
Preparation for cleaning/disinfection
Directly after each treatment, all parts of the INQUA®Profi nebuliser must be
cleaned of medication residue and contamination.
In order to help reduce water pollution, do not dispose of the medication res-
idue in the waste water system. Wipe it up with tissues and dispose of the tis-
sues with non-recyclable waste.
ÖIf this has not been done, disconnect
the connection tubing from the
INQUA®Profi nebuliser.
ÖDismantle the nebuliser into its indi-
vidual parts (nebuliser upper sec-
tion (1), nebuliser lower section (3)
and mouthpiece (2)).
1
2
3

34
Cleaning with warm tap water
ÖClean all parts of the nebuliser thoroughly for 5 minutes with warm tap wa-
ter (approx. 40°C) and a little dishwashing liquid with no skin protecting or
disinfecting additives (dosage according to the manufacturer's recom-
mendations) until all residues of the inhalation solution and other contam-
inants have been removed.
ÖThen rinse all parts thoroughly under warm running water (approx. 40°C
without dishwashing liquid).
ÖPour out the water that has collected in the individual parts.
You can remove excess water more quickly by shaking the parts.
Disinfection
Disinfect the dismantled nebuliser after cleaning.
Disinfection with hot steam
The INQUA®Profi nebuliser can be disinfected with the NUK Vaporisator 6
(NUK item no. 10.251.010) or the Petra disinfector DI 6.00
(item no. 0229 0000). To ensure that the hot steam passes through all the in-
dividual components of the nebuliser, the components must be placed upright
in the disinfector.
Be sure to follow the instructions for use of the NUK Vaporisator 6 and the
DI 6.00 disinfector.
Possible alternative: Disinfection with boiling water
ÖPlace the individual parts of your INQUA®Profi nebuliser in boiling water
for at least 15 minutes.
When boiling, make sure that there is enough water in the pot to prevent the
nebuliser parts from coming into contact with the base of the pot.
WARNING: Risk of fire if boiling operation is not supervised!
As soon as the water has evaporated completely, the plastic in the
pot will start to melt. If the heat source is not cut off, the plastic will
decompose, giving off smoke, and may cause a fire.
Never leave the boiling procedure unattended!

en
35
Drying
ÖPlace the INQUA®Profi nebuliser components on a dry, clean and ab-
sorbent surface.
ÖLet them dry completely.
The components should not be dried in damp rooms (e.g., the bathroom). The
drying process may be accelerated by using a hair dryer.
Storage
ÖBetween uses, and in particular during long breaks in treatment, wrap the
dry nebuliser in a dry, clean, lint-free cloth (e.g., a tea-towel) and keep it
in a dry, dust-free environment. Or pack the nebuliser or its individual
parts in the storage compartment on the INQUA®compressor.
CAUTION:
If you use a hairdryer, please hold it at least 15cm away from the
parts.
WARNING: Bacterial contamination caused by residual
moisture
Inadequate drying can present a risk to health, e.g., by causing an
infection.
Make sure that all components are free of residual moisture before
reassembling them and/or stowing them in the storage compart-
ment, or using them for inhalation again. All components must be
perfectly dry.
WARNING: Danger to health from choking or misuse
If children are allowed unsupervised access to the inhalation device,
there is a danger that they may swallow small parts or injure them-
selves by using the device incorrectly.
Keep the INQUA®Profi nebuliser out of children's reach.

36
Care of the connection tubing
ÖAfter inhalation, check the connection tubing (4) for condensation. To do
this, after each treatment pull the tubing off of the nebuliser and allow the
INQUA®compressor to continue running until all moisture has been re-
moved by the airstream.
ÖDo not use water or any other cleaning or disinfecting solutions to clean
the connection tubing.
Replace the tubing if it is contaminated.
WARNING: Bacterial contamination caused by residual
moisture
Any residual moisture in the connection tubing can present a risk to
health, e.g., by causing an infection.

en
37
MISCELLANEOUS
Storage and transportation conditions
Do not store the INQUA®Profi nebuliser in damp rooms (e.g., bathroom). Do
not store or transport it together with damp objects.
Protect the INQUA®Profi nebuliser from continuous, direct sunlight while it is
being stored and moved.
In general, the nebuliser should be stored as described in Chapter "Storage",
page "35" between applications.
Material resistance
Do not expose the nebuliser to temperatures above 137°C. In general, like all
plastic parts, nebulisers are susceptible to wear. The nebuliser can be disin-
fected up to 300 times, but then it must be replaced. Even if the nebuliser has
been disinfected fewer than 300 times, it must still be replaced after one
year's use.
Disposal
The components of the INQUA®Profi nebuliser and the replacement set must
be disposed of with non-recyclable waste.

38
SPARE PARTS/ACCESSORIES
TECHNICAL DATA
General
Recommended nebuliser gases: Air
Minimum flow: 3.0l/min
Corresponding operating pressure: 50kPa (0.5bar)/7psi
Maximum flow: 6.0l/min
Corresponding operating pressure: 200kPa (2.0bar)/29psi
Minimum fill: 2ml
Maximum fill: 8ml
Item description Item no.
INQUA®Profi nebuliser set
Contents: INQUA®Profi nebuliser with mouthpiece, child
mask, air filter, adult mask, connection tubing, removal tool
for changing the filter
BR021100
Table of contents
Other INQUA Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Alecto
Alecto ACS-31 manual

Apollo Healthcare Technologies Limited
Apollo Healthcare Technologies Limited APH040 user manual

HEINE
HEINE iC 2 manual

Smiths Medical
Smiths Medical BCI 3304 Service manual

Quantum
Quantum QW-400 Series Installation and operation manual

Ece Medical
Ece Medical Respirox T-30T plus manual