3
DEVICE DESCRIPTION
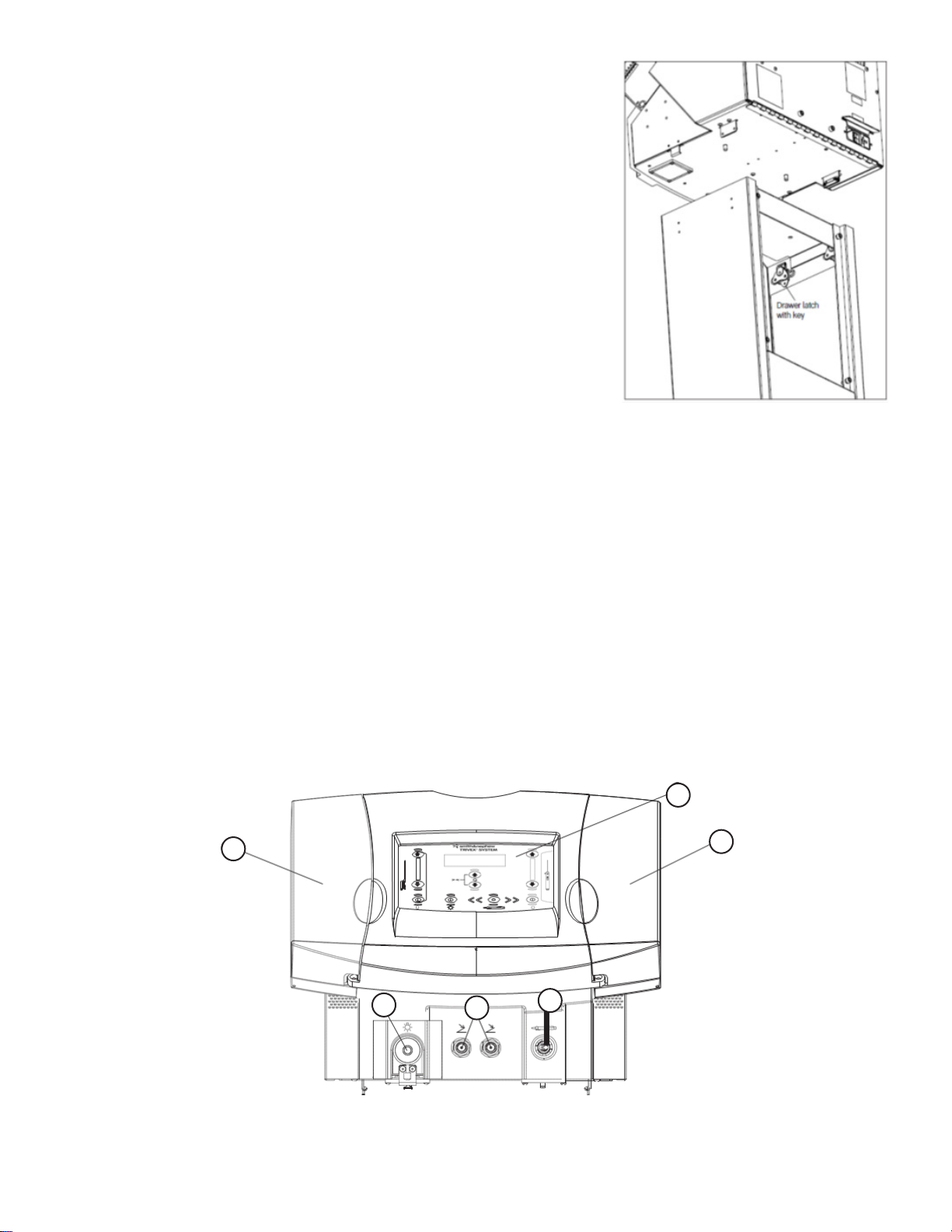
TheTRIVEX System provides controls for the operation of the light source for the Illuminator, the tumescent pump, the saline pump, and the mode and variable
speed operation of the Resector Handpiece. The TRIVEX System uses a pair of peristaltic pumps to provide irrigation and tumescence anesthesia for the
Transilluminated Powered Phlebectomy procedure.The left hand pump is dedicated to providing the tumescence anesthesia via the TRIVEX System Illuminator.
The right hand pump is dedicated to providing saline to the TRIVEX Resector Handpiece for resector tip irrigation.TheTRIVEX System uses a metal halide arc lamp
to provide intense, white light via the TRIVEX System Illuminator for transillumination.The illuminator connects with ber optic cables to the TRIVEX System to
provide transillumination during endoscopic resection of supercial varicosities of the lower extremities. The Resector Handpiece drives the Resector and features
push-button controls for Resector operation.The Cart provides a mobile base for the Control Unit and a mast for hanging the saline and tumescent solution
irrigation bags.The Footswitch causes air pressure to activate a switch inside the Control Unit which turns on the tumescence pump.
TheTRIVEX System is intended for use by vascular or general surgeons for treatment of patients of all ages and sexes that require removal of varicose veins.
INDICATIONS FOR USE
TheTRIVEX System is indicated for use in ambulatory phlebectomy procedures for the resection and ablation of varicose veins.
CONTRAINDICATIONS
Use of theTRIVEX System is contraindicated in situations where ambulatory phlebectomy is contraindicated.
WARNINGS
Please read this manual before using the TRIVEX System.The brief operating instructions in this guide will make the system easier to use, while the
recommended service and maintenance procedures will ensure optimal performance and reliable use. As with any surgical instrument, there are important
health and safety considerations. These are listed below and reiterated within the text.
Prior to using theTRIVEX System, it is essential that all components of the system be inspected for damage that can negatively impact the equipment
performance.The inspection should include all equipment to be used in surgery, including the illuminator, handpiece, cables, and accessories.
When removing the TRIVEX System and accessories from the shipping container, inspect contents to ensure that all components from the“Unpacking the
Components”section are available.
Contact your LeMaitreVascular Representative if damage is noted.
• Before using theTRIVEX System for the rst time, you should review all available product information. Surgeons should become familiar with this surgical
technique and theTRIVEX System. You should be experienced in ambulatory phlebectomy surgery using powered instruments. Healthy tissue can be
injured by improper use of theTRIVEX System resector. Use every available means to avoid such injury.
• TRIVEX Resector Kits are packaged as a set.They must be used as supplied. Do not interchange resector components.
• Illuminator inow tube sets and resector kits are provided STERILE and are for single use only. Do not reuse. Do not resterilize. Prior to use, inspect the
product package for signs of damage or tampering. Discard any opened and unused product. Do not use after the expiration date.
• Resterilization and/or re-use of the Illuminator Tube Sets or Resector Kits may cause mechanical damage to these products. This may result in injury to the
patient or user.
• TheTRIVEX System pumps should not be running while setting up the tubing. Injuries to the operator’s hands can occur.
• Work exclusively with sterile substances, sterile uids, and sterile accessories.
• This system is intended only for use with exible uid containers and uid bags. Glass containers or bottles may break, and there is a risk of implosion.
• Use of bags or containers not approved for this system, or large and/or lopsided loads, may cause the device to tip over.
• If visualization is lost during any point in the procedure stop resecting immediately.
• Excessive pressure of theTRIVEX resector against the vessel or prolonged activation of theTRIVEX resector in a stationary position may result in perforation
of the resector through the limb surface.
• Do not hold the light source shutter open without a ber optic cable in place. Failure to observe this precaution may result in eye injury.
• During procedure, avoid prolonged contact of the Illuminator tip to patient tissue or ammable materials.The Illuminator tip may reach high temperatures
due to high-intensity light transmission.
• DANGER: Risk of explosion if used in the presence of ammable anesthetics.
• When the light source is turned on, do not look directly at the metal halide arc lamp without protective goggles.
• To prevent electrical shock, do not remove theTRIVEX System console cover. There are no user-serviceable components inside. Dismantling the equipment
will void the warranty. Refer servicing to LeMaitreVascular.
• To prevent electrical shock, connect the power cord to a properly-wired grounding receptacle only.
• To prevent electrical shock, unplug the unit from the electrical outlet before attempting to replace the fuses.
• Use extreme caution:The high internal pressure of the lamp may cause an explosion, regardless of whether the lamp is cold or hot. Always wear protective
clothing and a face mask when handling the lamp.
• Hazardous high voltage and energy are present at the output and in the internal circuitry of this unit.
• If this unit is congured as part of a system, the entire system should be tested for compliance with IEC 60601-1.
• If the leakage current of the congured system exceeds the limits of IEC 60601-1, install an appropriately rated UL 60601-1/IEC 60601-1 approved isolation