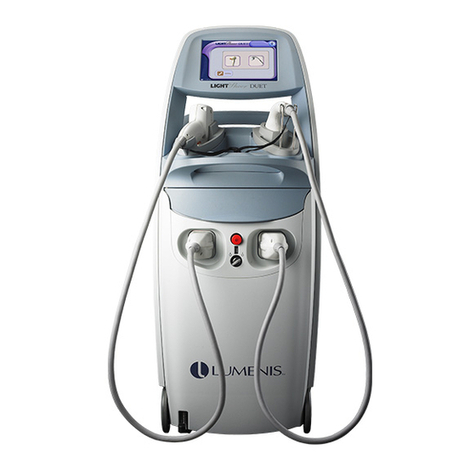
This manual is copyrighted with all rights reserved. Under copyright laws, this manual may not be copied in
whole or in part or reproduced in any other media without the express written permission of Lumenis. Permitted
copies must carry the same proprietary and copyright notices as were affixed to the original. Under the law,
copying includes translation into another language.
Please note that while every effort has been made to ensure that the data given in this document is accurate, the
information, figures, illustrations, tables, specifications, and schematics contained herein are subject to change
without notice.
Lumenis, the Lumenis logo, UltraPulse, UltraPulse SurgiTouch, SurgiTouch+, Encore, UltraScan CPG,
PigmentFX, ActiveFX, ActiveFX gentle, MaxFX, DeepFX, SCAAR FX, PreciseFX, IncisionFX, BrowFX,
CoolScan, CO2Lite, TrueSpot, ClearSpot, UltraFlex, Lumenis/Nezhat, AcuSpot, OtoScan, OtoLAM, AcuBlade,
BeamAlign, MicroSlad, and FiberLase are trademarks or registered trademarks of Lumenis.
Retin-A and Renova are registered trademarks of Ortho Pharmaceuticals Corporation, Dermatological Division.
Melanex is a registered trademark of Neutrogena Dermatologics. Valtrex is a registered trademark of Glaxo
Wellcome, Inc. Famvir is a registered trademark of SmithKline Beecham Pharmaceuticals. Cipro is a registered
trademark of Bayer Corporation. Coppertone and Shade are trademarks, distributed by Schering-Plough
HealthCare Products, Inc. Vaseline is a registered trademark of Chesebrough-Pond’s USA, Co. Acutane is a
registered trademark of Roche Laboratories, Inc.
Copyright© Lumenis Ltd.
May 2012
0637-129-01
Revision G
Manufactured by Lumenis Ltd.
P. O . B . 2 4 0
Yokneam 20692
Israel
Directive 2002/96/EC on Waste Electrical and Electronic Equipment (WEEE)
In accordance with Directive 2002/96/EC on Waste Electrical and Electronic Equipment (WEEE), any
item which is marked with the crossed-out wheelie bin symbol must not be disposed of as unsorted
municipal waste, but segregated from other waste types for eventual treatment and recovery at an
approved recycling facility.
By returning waste electrical and electronic equipment via the correct segregated disposal channel, users
can ensure the environmentally sound treatment and disposal of the waste equipment, thereby reducing
the potential for any environmental or health risks that could arise as a result of incorrect disposal.
Lumenis provides web-based collection, recycling and reporting arrangements to the business end-user
for equipment marked with the crossed-out wheelie bin. Please visit www.lumenis.com/recycling to
understand what arrangements Lumenis has made in each EU Member State.
Authorized Representative:
Lumenis GmbH Germany
Heinrich-Hertz-Strasse 3
D-63303 Dreieich-Dreieichenhain
Germany