MMO1001 rev.1 -19/01/2016 5- 58
1.1
Desc
riptio
n of
the
EM
devi
ce
DESCRIPTION OF THE EM DEVICE
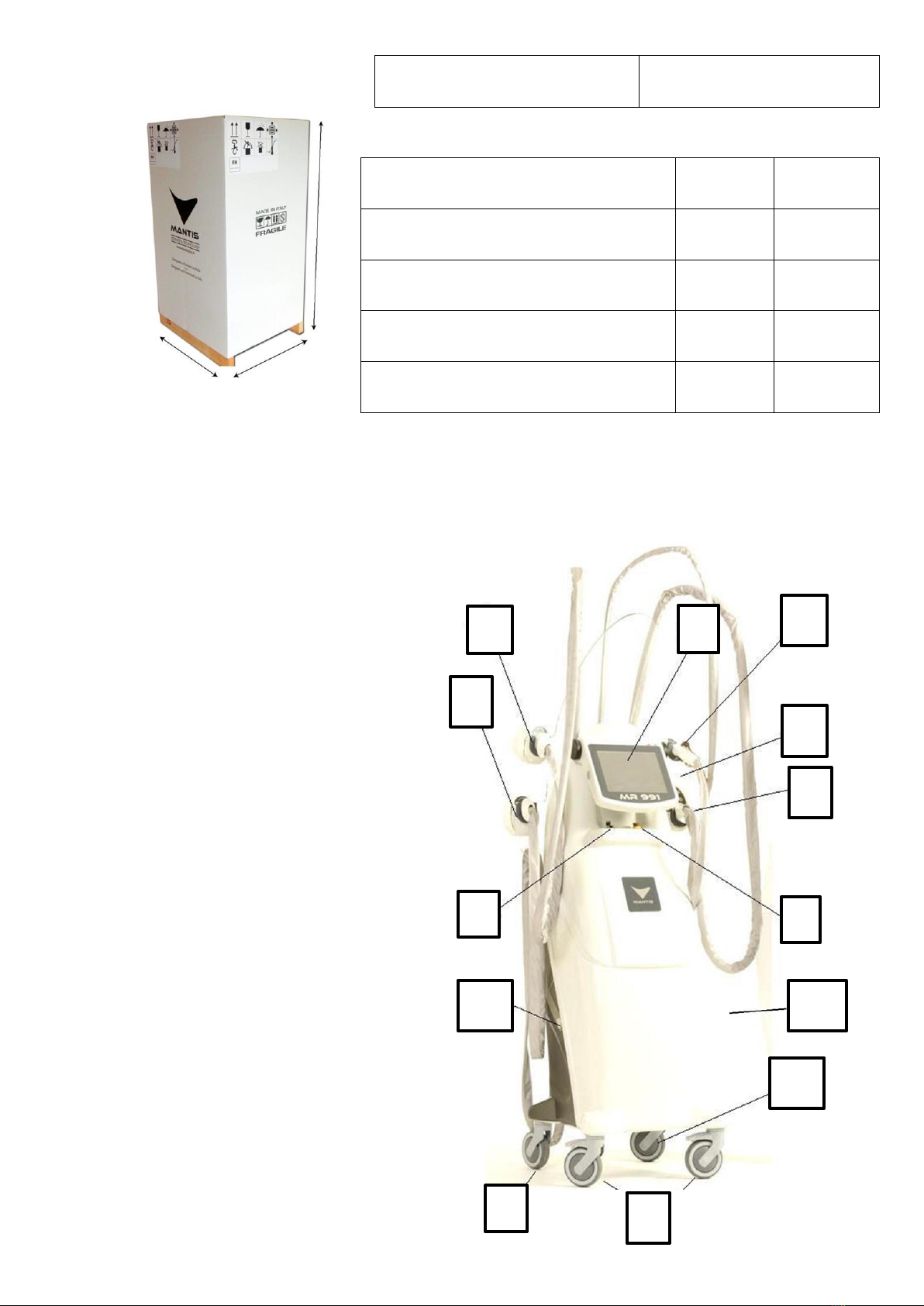
MR991 is an EM device that combines the following three technologies, which can be
easily programmed by the operator:
MRM - mechanical manipulation with motorised rollers - for effective endomassage with
mechanical rollers and micro-suction.
PMFS SYSTEM -pulsed magnetic fields with stochastic resonance- the use of which has
shown that treated areas, based on tests carried out, have a greater blood supply than
usual, thus leading to greater oxygen exchange with the tissues and at the same time
draining all toxic and unwanted substances towards the organs of detoxification and
excretion.
Thanks to this detoxifying action, which drains the stagnant liquid in the tissues, the
PMFS system is ideal to treat lymphoedema and E.F.P.
PMF, using high energy magnetic fields, has a specific effect on ions, macromolecules
and membranal proteins.
DEFINITIONS AND SYMBOLS USED IN THE MANUAL
The manual defines some figures authorised to operate on the EM device in different
manners:
Manufacturer: the producer of the EM device who affixes their own mark on the EM
device itself (MANTIS s.r.l.).
Operator: personnel who use the EM device, who has working knowledge of its operation
following a course with subsequent authorisation granted by the manufacturer. The
operator is also in charge of cleaning and routine maintenance.
Customer: the person who avails of the treatment or who requests it. Technical
assistance: personnel in charge of special maintenance and repairs of the EM device,
specifically assigned by the manufacturer.
The following symbols are used in the manual:
Important requirements concerning safety and operation of the
device