Physio Control LIFEPAK 15 User manual
Other Physio Control Medical Equipment manuals

Physio Control
Physio Control LIFEPAK 15 V4 User manual
Physio Control
Physio Control LIFEPAK 15 Quick start guide

Physio Control
Physio Control LIFEPAK CR PLus User manual

Physio Control
Physio Control LIFEPAK CR PLus User manual
Physio Control
Physio Control LIFEPAK 20 User manual

Physio Control
Physio Control LPCR2 User manual

Physio Control
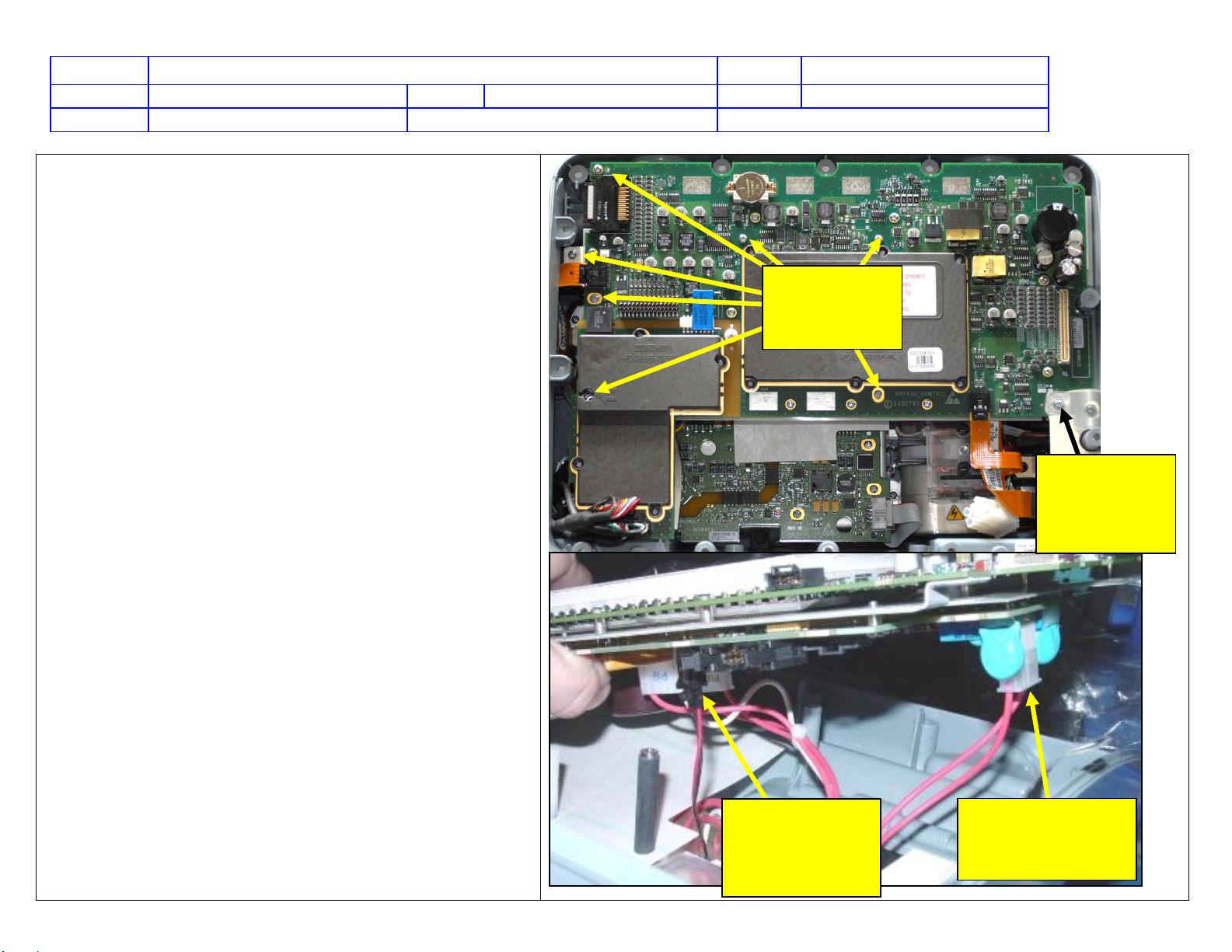
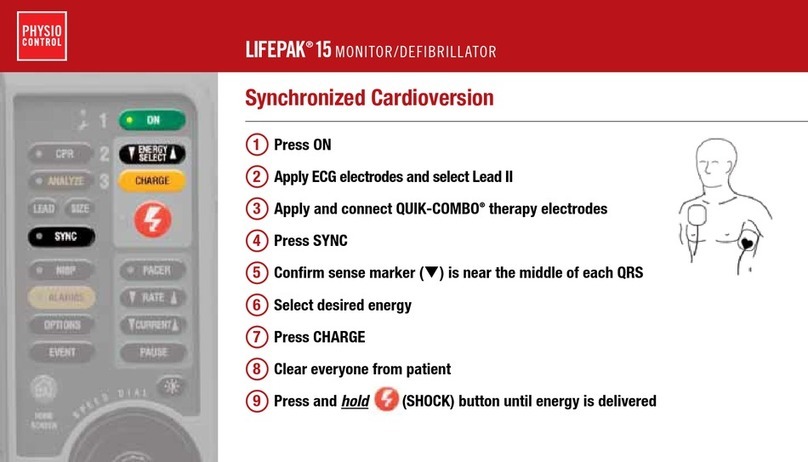
Physio Control LIFEPAK 15 Technical manual

Physio Control
Physio Control LIFEPAK CR PLus User manual

Physio Control
Physio Control Lifepak CR2 LIFELINKcentral User manual
Physio Control
Physio Control LIFEPAK 15 User manual

Physio Control
Physio Control Lifepak 1000 User manual
Physio Control
Physio Control LIFEPAK 15 User manual
Physio Control
Physio Control LIFEPAK 15 User manual

Physio Control
Physio Control LIFEPAK 20 User manual

Physio Control
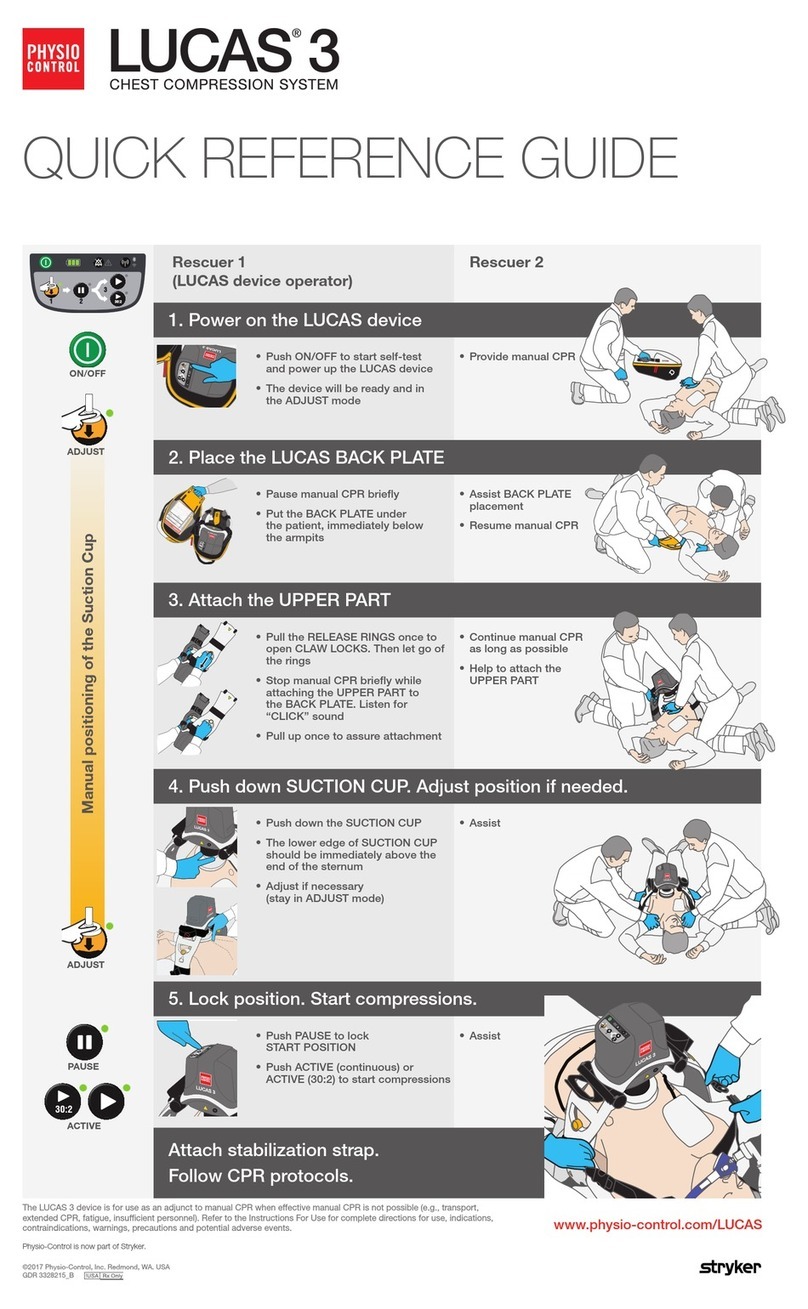
Physio Control LUCAS 3 User manual

Physio Control
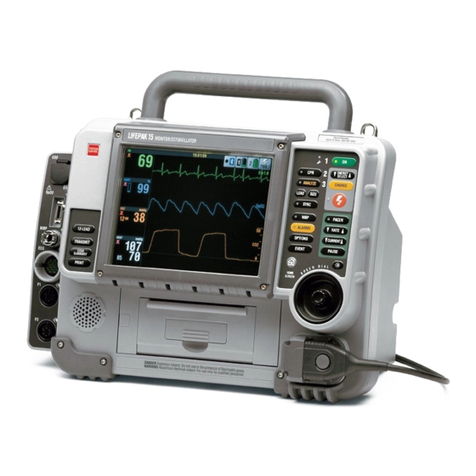
Physio Control LIFEPAK 500 User manual
Physio Control
Physio Control LIFEPAK 15 User manual
Physio Control
Physio Control LIFEPAK 15 V4 User manual

Physio Control
Physio Control LIFEPAK 12 User manual

Physio Control
Physio Control lucas 2 User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual