2
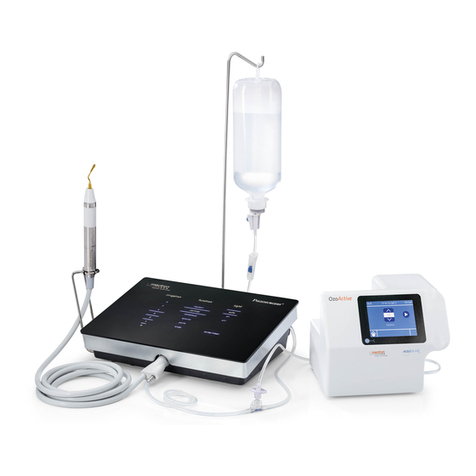
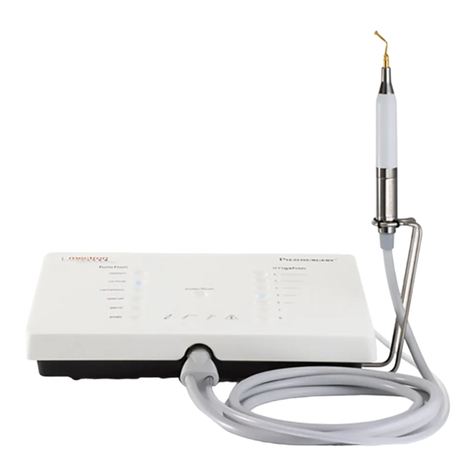
1.2 DescriptionoftheDevice
Theuserinterfacehasbeenoptimizedwiththe
PIEZOSURGERY®exmakingallthefunctions
readily available by integrating them in the
touchkeyboard.
The PIEZOSURGERY®ex is a device that
uses ultrasonic piezoelectric technology to
generate mechanical microvibrations of the
inserts, to eectively cut mineralized tissues.
Thisallowsanecientandsafecuttingwhich
preserves the integrity of the osteotomized
surfaces.
The micrometric, ultrasonic vibrations of
the inserts provide greater precision and
a selective cutting action compared to
traditional methods such as drills or oscillating
saws (which act with macrovibrations),
thereforeminimizingtraumaticeectonsoft
tissues.
Thecavitationeectoftheirrigatingsolution
helpstokeeptheoperatoryeldblood-free.
This provides an optimal intra-operatory
visual control thus increasing safety, even in
areas that are anatomically most dicult to
access.
This medical device can be used on any
patient of any age, weight, height, gender and
nationality.
1.2.1 PatientGroupDirections
This medical device is designed to be used with the following patient population:
• Infants;
• Children;
• Adolescents;
• Adults;
• Elderly.
This medical device can be used on any
patient of any age, weight, height, gender and
nationality.
1.2.2 PatientSelectionCriteria
The use of the device is not recommended in
the following cases:
1. Patients with active implantable
medicaldevices(forexample:
pacemakers,hearingaidsand/orother
electromagnetic prostheses) without
thepriorauthorizationoftheirdoctor;
2. Women who are pregnant or
breastfeeding, due to the restrictions
associated with the possible use of
medical solutions such as anaesthetics
3. Patients with allergies;
4. Patients with pathologies or clinical
conditions for which it is not
recommended to perform surgery
or for which it may represent a
contraindication according to the
treating physician. These conditions
may include, but are not limited to:
heart disease, diabetes, cirrhosis, HIV
infection, pregnancy or breastfeeding,
radiation therapy, chemotherapy,
immuno-suppressivetherapy,allergies
and psychiatric disorders;
5. Patients with unsuitable treatment sites.
All models of “Piezosurgery” bone surgery
devices are intended for professional use only.
Therefore, the user is the only person able to
decide if and how to treat their patients.
1.2.3 IndicationsforUse
The use of the device is suitable for all the appropriate patients (see chapter above) for whom a
bone surgery treatment is prescribed, by the treating physician, within the intended use of the
device (see
Chapter 1.1 on page 1
).