L03030 1.0 03/2018 4
Table of Contents
Part A –WARNINGS, INTRODUCTION AND INSTRUCTIONS............6
SECTION A1: Important Labels, Symbols and Warnings .........................................................6
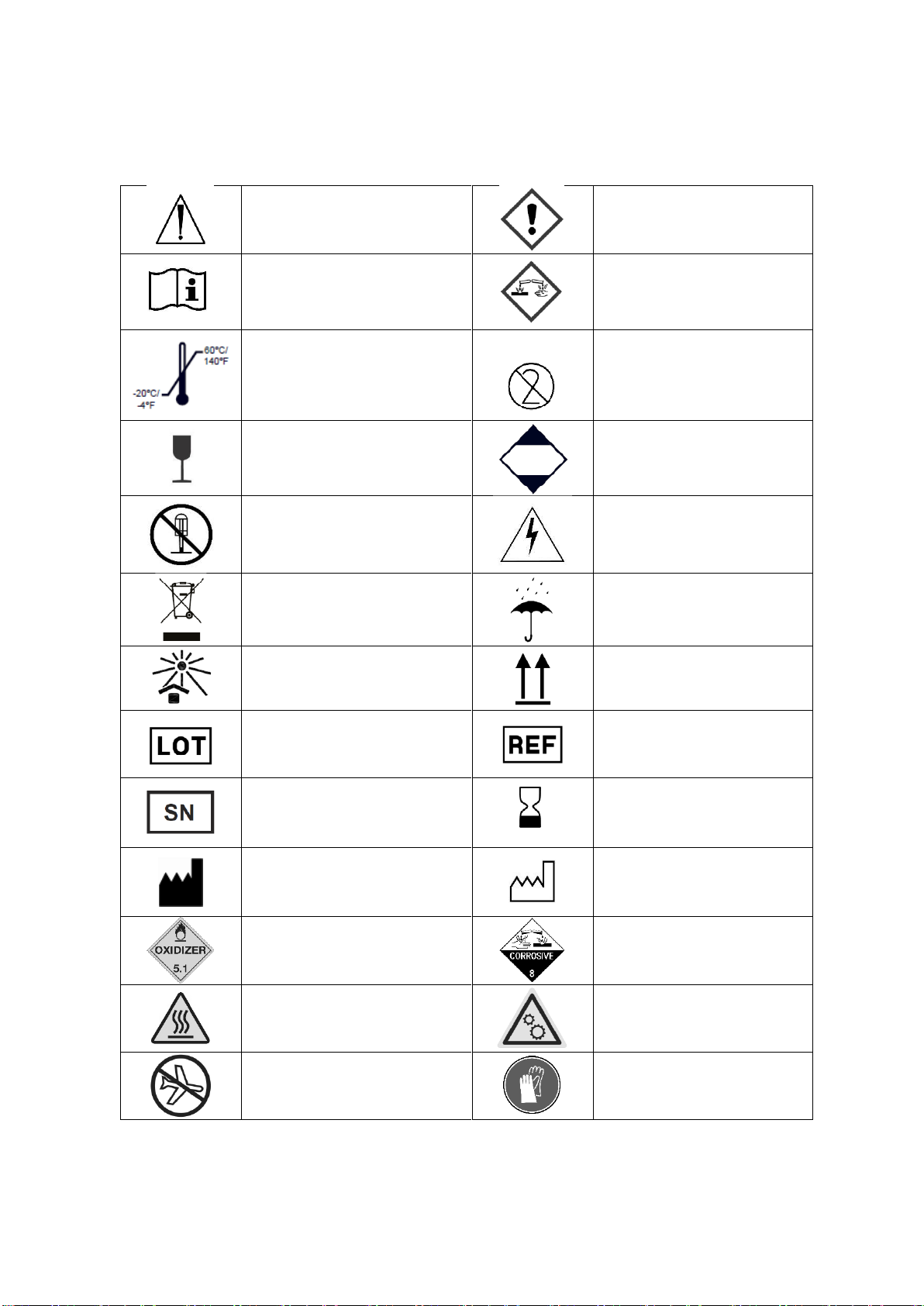
A1.1 Labels and Symbols.............................................................................................................6
A1.2: Warnings................................................................................................................................7
SECTION A2: Introduction to the trophon2................................................................................8
A2.1 Indications for Use..................................................................................................................8
A2.2 Disinfection Process...............................................................................................................8
A2.3 Validated Probes, Disinfectants and Chemical Indicators......................................................8
A2.4 Training...................................................................................................................................8
A2.5 Environment and User Profile.................................................................................................9
SECTION A3: Instructions............................................................................................................9
Part B –SET UP..................................................................................10
SECTION B1: trophon2 Overview..............................................................................................10
B1.1 trophon2 Features ................................................................................................................10
B1.2 Cable Tray............................................................................................................................11
SECTION B2: Installation Guide................................................................................................12
B2.1 Positioning your trophon2.....................................................................................................12
B2.2 Powering On.........................................................................................................................13
B2.3 Initial Setup...........................................................................................................................13
B2.4 Warm up Cycle.....................................................................................................................13
B2.5 trophon2 Touch Screen........................................................................................................13
B2.6 Basic Settings.......................................................................................................................13
B2.7 AcuTraceTM ...........................................................................................................................13
B2.8 AcuTraceTM Settings.............................................................................................................14
SECTION B3: trophon AcuTrace PLUS.....................................................................................15
B3.1 Activation ..............................................................................................................................15
B3.2 Network Parameters Setup ..................................................................................................15
PART C –OPERATION.......................................................................15
SECTION C1: Loading the Disinfectant Cartridge...................................................................15
SECTION C2: Logging the trophon Chemical Indicators........................................................15
SECTION C3: Routine High Level Disinfection Cycle .............................................................15
C3.1 Preparing the Probe .............................................................................................................15
C3.2 Inserting the Chemical Indicator...........................................................................................16
C3.3 Positioning the Probe ...........................................................................................................16
C3.4 Closing the Chamber Door...................................................................................................18
C3.5 Disinfecting the Probe ..........................................................................................................18
C3.6 Removing the Probe.............................................................................................................19
C3.7 Sleep Mode ..........................................................................................................................19
PART D –RECORDS...........................................................................19
SECTION D1: Record Options ...................................................................................................19
PART E –MAINTENANCE AND ROUTINE CARE...............................20
SECTION E1: Preventative Maintenance Service ....................................................................20
SECTION E2: Purge Cycle..........................................................................................................20
E2.1 When to Run a Purge Cycle.................................................................................................20
E2.2 How to Initiate a Purge Cycle ...............................................................................................20
SECTION E3: Regular Cleaning.................................................................................................21
SECTION E4: Transporting the trophon2 .................................................................................21
SECTION E5: Disposal of trophon2 ..........................................................................................21
PART F –TROUBLESHOOTING..........................................................21
SECTION F1: Incomplete or Failed Cycles...............................................................................21