New Age MAGNETER PRO User manual

MAGNETER PRO
USER MANUAL
- Instructions -
New Age Italia srl
Via De Brozzi, 3 - 48022 Lugo (RA)
Tel.:+39-0545.32019 - Telefax: +39-0545.369028
Web: www.newageitalia.it - E-mail: info@newageitalia.it
This document is of ownership of the New Age Italia srl. All the rights are reserved. And' forbidden the
copy and the reproduction with any mean, included the total or partial photocopy of the content without
authorization written of the New Age Italia srl.

2/18

3/18
INDEX
Cap.1 –PRESENTATION......................................................................5
–What is MAGNETER PRO ................................................................5
–What is MAGNETER PRO for ? .......................................................5
–Who can use MAGNETER PRO ? ....................................................5
Cap.2 –INDICATIONS AND CONTRAINDICATIONS.......................6
–Indications........................................................................................6
–Contraindications ............................................................................6
Cap.3 –OPERATIONS...........................................................................8
–Solenoids' connection and application.........................................8
–Turn on the device...........................................................................9
–Program selection ...........................................................................9
–Starting the therapy........................................................................9
3.1.1 - Manual mode.................................................................................9
3.1.2 - Preset program mode...................................................................9
3.1.3 - Personal program mode............................................................ 10
–Setting the intensity......................................................................10
–End or interruption of the session...............................................10
–Turn off the device........................................................................10
Cap.4 –PRESET PROGRAMS............................................................11
Cap.5 –APPLICATIONS.....................................................................13
–Therapy planning ..........................................................................13
–Intensity regulation.......................................................................13
–Body position during the sessions ..............................................13
Cap.6 –POWER SUPPLY....................................................................14
Cap.7 –SYMBOLS...............................................................................14
Cap.8 –MAINTENANCE.....................................................................14
–Solenoids, band, cushion and carpet ..........................................14
–Device and power supply cable ...................................................14
–Feeder substitution .......................................................................14
–Immediate maintenance...............................................................15
Cap.9 –WARNINGS ............................................................................15

4/18
Cap.10 –TECHNICAL FEATURES....................................................17
–Power supply..................................................................................17
–Output features .............................................................................17
–Other features................................................................................17
Cap.11 –ENDOWMENT OF BASE / ACCESSORIES........................18
–Endowment of base.......................................................................18
–Accessories and consumed materials..........................................18
Cap.12 –BIBLIOGRAPHY..................................................................18
Model: Magneter PRO .....................................................................19

Rev. 01 del
25/06/14
5/21
Ca p.1 –PRESENTATION
MAGNETER PRO belongs to MODUL LINE, the new type of electromedical
instruments for physiotherapy, that can combined together up to 3 different therapies
in the same machine. The reduced dimensions, the facility of use and the versatility
are the principal characteristics of this innovative line of products.
–What is MAGNETER PRO
The constant search in the sector of the medical devices has brought to the creation
of the new system for magnetotherapy, constituted by the generator MAGNETER
PRO. The innovative system of the software, electronic cards of small dimensions
inserted inside the instrument, allow the generation of magnetic fields to therapeutic
purpose for the treatment of common pathologies (lesions, accidents, illnesses)
through the use of preset programs ready to use.
The possibility to adjourn the device and the programs, the technological innovation
and the facility of employment make it an extremely versatile and innovative
product in the electromedical instruments field.
–What is MAGNETER PRO for ?
With MAGNETER PRO it is possible to apply magnetic fields with benefits effects
on the reconstruction of the bones and, in general, for the regeneration of damaged
tissues. Checking the effects and the course of the therapy, it is possible to suit the
therapy for the demands of the patient.
–Who can use MAGNETER PRO ?
MAGNETER PRO finds in the medical field (physiotherapy particularly) the fittest
environment to express completely his/her own potentialities. Nevertheless, for the
simplicity of use, it is usable, over that from physicians and therapists of the
rehabilitation, also from who desires to use it at home, thanks to the immediateness
and versatility of the product.
ATTENTION: for home use follow carefully the indications of the therapist.
ATTENTION: READ CAREFULLY
THE INSTRUCTIONS BEFORE USE

Rev. 01 del
25/06/14
6/21
Ca p.2 –INDICATIONS AND CONTRAINDICATIONS
–Indications
The pathologies most common that are taken care of with the magnetic fields they
are those to load of the apparatus osteo-muscle-tendineo and, particularly they are
suitable in the treatment of:
periostitis;
tenonitis;
arthrosis;
muscular contractures;
cicatrizations;
organized oedemas.
Besides, the magnetic fields preserve today also one born validity in the treatment of
rheumatologic abarticular alterations as the shoulder periarthritis, the epicondylitis
of the elbow and other pathologies, thanks to the analgesi, fybrolitic and
decontracturant effect.
The interested structures mainly suffer the most greater traumas in the physical
activity but, in many cases, they can also be caused by the aging (rheumatisms,
muscular ipotrofia, lack of equilibrium for the scarce movement); these cases, are
particularly in strong increase for progressive rising of the middle age, not followed
by a suitable improvement of the quality of the life.
In the sporting disciplines the most frequent traumas strike the articulations of the
knee and the ankle and they can be treated with the magnetic fields through of the
daily and repeated applications for a certain number of sessions. The treatments are
effected above all to analgesic purpose propedeutico to the sessions of kinesitherapy.
–Contraindications
The magnetic fields have the same side effects of the phisic therapies that act with
the endogenous production of heat:
degenerative osteoporosis;
presence of metallic fragments;
varicose veins;
thrombosis and acute thromboflebitis;
arteriopathologies with hemorrhages;
menstruations;
tumors;
tuberculosis;
acute inflammatory trials;
cutaneous lesions;

Rev. 01 del
25/06/14
7/21
alterations of the sensibility.
In the applications of magnetic fields, the use is dissuaded on:
specialized tissues (fertile metafisis, testicles, ovaries), because they can be
damaged;
avoid the use in cardiac area and, particularly, in presence of electric equipments
(pace-maker), because it can cause permanent damages.
NOTES:
Particular precautions must be adopted when applications are effected on the
back bone of patients, with diagnosis of laminectomia, for possible damages to
the bone marrow.
The applications cannot be effected in the immediate proximities of the ocular
globes and the uterus (abdomen / lumbar zone) in case of pregnancy for the risk
to provoke the phenomenon of the cavitation, dosing hips corrected.
The presence of articular prosthesis and a halves of metallic synthesis is
problematic because they can absorb a superior quantity of magnetic fields and
be deteriorated or cause damages to the adjacent structures.
The use of this physical therapy is dissuaded the treatment of pathologies of the
juvenile age and, above all, childish (ex. fertile cartilages of conjugation).
Rev. 01 del
25/06/14
8/21
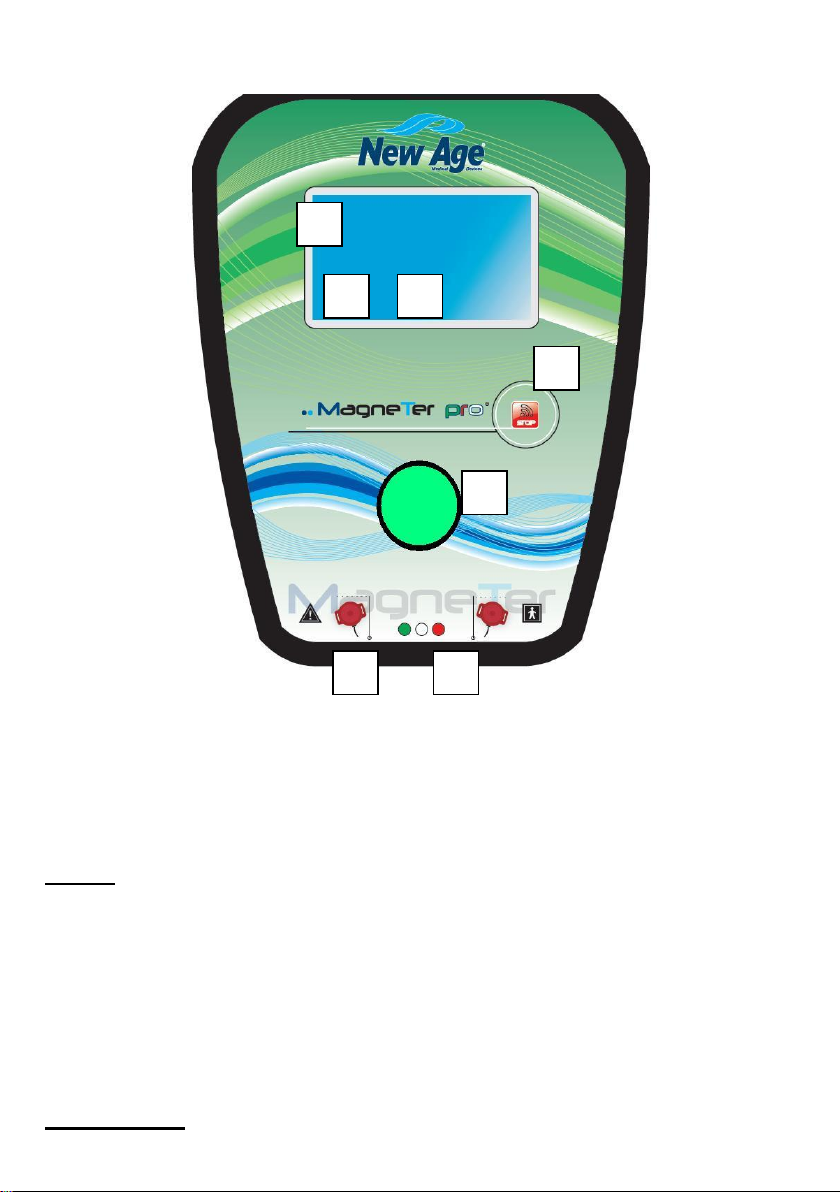
Ca p.3 –OPERATIONS
1 –Display TOUCH SCREEN 6 –START/PAUSE
2–STOP BUTTON 7 –PROGRAMS
3–KNOB + PUSH BUTTON
4–OUTPUT 1
5–OUTPUT 2
NOTE: before applying the magnetotherapy check the side effects and consult
a physician.
–Solenoids' connection and application
Connect the cable of the solenoids to the front output of the instrument. Insert the
connector by rotating till the connections coincides with the output; screw until the
connector fix tight. To use the rubber solenoids, apply them over the zone to treat
and fix them with the elastic bands.
ATTENTION: when you apply two solenoids one in front of the other (ex.
1
2
3
4
5
6
7
Rev. 01 del
25/06/14
9/21
Joints like elbow, knee, ankle), place on the skin the same draw (spiral) so that the
magnetic fields of the two solenoids is added.
To use cushion and carpet (not included), just apply over them the interested zone
(ex. head, shoulders, back, basin, feet, etc.) or the whole body; the magnetic band
(not included) can be used: closed like a ring for the limbs (arms, legs, joints) or
open and applied above the treating zones. To perform correctly the applications
follow the indications of the Chap. APPLICATIONS and see the attached photos.
NOTE: if the carpet (not included) is connected to one channel, it is not possible to
connect other solenoids into the other channel (ERR4).
–Turn on the device
To turn on MAGNETER PRO press 1 on the rear key ON/OFF. The display shows
firm and device's name and places on the first program.
–Program selection
Select the program with DOWN/UP keys (see list of Chap. PRESET PROGRAMS).
–Starting the therapy
Press (start) to begin the therapy.
NOTE: do not press start with no solenoid connected to the device (the display
show the message "Insert solenoid 1 Insert solenoid 2"; after connecting one or two
solenoids, press start.
3.1.1 - Manual mode
Press START on the initial screen to access the manual mode area. In this area, by
TURNING THE KNOB you can vary the value of the parameter selected, whereas
by PRESSING THE KNOB you pass from one parameter to the next. The
concerned parameters are: power output of the ultrasound, duration, modulation,
frequency.
3.1.2 - Preset program mode
Press START on the initial screen (touch screen) to access the work area. Then
press PROGRAMS (on touch screen)to enter the Menu for choosing specific
protocols where first of all the therapy to be used is selected, by turning the encoder
and then pressing ENTER (touch screen) or else PRESSING THE KNOB to
confirm the program selected.
Rev. 01 del
25/06/14
10/21
3.1.3 - Personal program mode
The custom-made programs are at the bottom of the list of pre-set programs. Press
START on the initial screen (touch screen) to access the work area. Then press
PROGRAMS (on touch screen)to enter the Menu for choosing specific protocols.
At the bottom of the list first of all the therapy to be used is selected, by turning the
encoder and then pressing ENTER (touch screen) or else PRESSING THE KNOB
to confirm the program selected.
NOTE: if the ultrasound head cable is not inserted in the device, the display will
show “elettr. Dist”. When the cable is put in, press START and the program restarts.
. To go back to the previous Menu press PAUSE STOP.
–Setting the intensity
The intensity (G=Gauss) is preset but can be manually set with the encoder knob of
the used Channel. The minimum increase is of 1 G and the maximum is 200 G. Set
an intensity of 50 G for the briefer daily applications (30-60 minutes) and inferior to
50 G for a prolonged therapy (more than 60 minutes); in case of pain or elevated
heating in the treated zone it is necessary to decrease the intensity with the knob or
interrupt the therapy by pressing pause stop.
If only one output cable is connected to the device, the display show the message
"N.C." on the unused channel.
NOTES: the instrument recognizes the type of connected solenoids (it doesn't
recognize different solenoids from those suitable from the house builder). If the
greatest solenoids are connected (carpet or pillow - not inclusive) the preset and the
maximum intensity varies in the following way:
1) carpet: maximum intensity 600 G.
2) cushion: maximum intensity 300 G.
3) velcro band: maximum intensity 300 G.
–End or interruption of the session
To interrupt before the end press the key pause stop: once for a break (to continue
press start); twice to finish the program.
–Turn off the device
To turn off the instrument press 0 (zero) on the rear key ON/OFF.

Rev. 01 del
25/06/14
11/21
Ca p.4 –PRESET PROGRAMS
MAGNETER PRO has 72 different preset programs The programs are listed in the
following chart.
N°
Prog.
Program name
(on the display)
Output
(continuous/modul
ated)
Frequency
(Hz)
Preset
intensity
(G)
Timer
(min)
P.1
Fractures
Cont
40
50
35
P.2
Rec. Fract.
Cont
75
100
45
P.3
S Fractures
Cont
40
80
40
P.4
L Fractures
Cont
80
100
60
P.5
Consol.delay
Cont
12
90
60
P.6
Pseudoar.I
Cont
12
40
45
P.7
Pseudoar.A
Cont
12
80
55
P.8
Osteopor.I
Cont
10
60
50
P.9
Osteopor.A
Cont
20
100
60
P.10
Artrithis
Cont
10
40
35
P.11
Periarthritis
Cont
10
80
25
P.12
Arthropathy
Cont
40
70
45
P.13
S. Arthrosis
Cont
15
40
35
P.14
L. Arthrosis
Cont
40
80
50
P.15
Intercostal Algia
Cont
70
80
30
P.16
Reumatoid Arthrosis
Cont
50
100
30
P.17
Hips arthrosis
Cont
50
100
30
P.18
Shoulder Arthrosis
Cont
50
100
30
P.19
Dorsal Arthoris
Cont
50
100
30
P.20
Edemas
Cont
40
80
30
P.21
Brachialgia
Cont
80
100
30
P.22
Headache
Cont
50
100
30
P.23
Flebitis
Cont
20
60
25
P.24
Tingle hand/feet
Cont
30
40
20
P.25
Acute Coxoartr.
Cont
10
50
40
P.26
Chronic Coxoartr.
Cont
25
80
50
P.27
Chronic Ankle Spr.
Cont
30
75
35
P.28
Acute Ankle Sprain
Cont
60
100
45
P.29
Knee sprain
Cont
80
90
55
P.30
Dislocation
Cont
50
100
60

Rev. 01 del
25/06/14
12/21
P.31
Stiff neck
Cont
30
60
35
P.32
Whiplash injury
Cont
20
40
40
P.33
Loc. Contr.
Cont
30
50
45
P.34
Widespread Cont.
Cont
70
80
60
P.35
Contracture
Cont
20
60
40
P.36
Strains
Cont
80
90
50
P.37
Myositis
Cont
10
70
60
P.38
Tendinitis
Cont
30
100
30
P.39
Tenovagin.
Cont
10
80
35
P.40
Carpal Tunnel
Cont
12
60
25
P.41
Bursitis
Cont
40
90
40
P.42
Chronic Epicond.
Cont
20
60
30
P.43
Acute Epicond.
Cont
40
80
40
P.44
Foot Tendonitis
Cont
70
90
30
P.45
Cervical
Cont
15
40
50
P.46
ChronicvLumbalgo
Cont
20
70
45
P.47
Acute Lumbalgo
Cont
40
90
55
P.48
Sciatalgia
Cont
50
100
60
P.49
Skin lesions
Cont
35
80
40
P.50
Loc. Bed sores
Cont
10
60
45
P.51
Widespr. Bed sores
Cont
25
85
60
P.52
Acne
Cont
10
50
30
P.53
Psor. Ecz.
Cont
15
60
35
P.54
Scars
Cont
30
40
40
P.55
Dermatitis
Cont
15
50
35
P.56
Inf. Urog.
Cont
12
60
55
P.57
Menstrual probl.
Cont
10
50
50
P.58
Haemorrhoids
Cont
20
80
45
P.59
Arteriop.
Cont
50
50
60
P.60
MigraineHeadache
Cont
12
40
30
P.61
Toothache
Cont
15
40
30
P.62
Diabetic Ulc.
Cont
12
90
60
P.63
Metatarsalgia
Cont
70
50
50
P.64
Vascular Pathologies
Cont
50
30
30
P.65 R
Consol. Delay 4h
Cont
12
30
4h
P.66
Pseudoarthros.4h
Cont
12
30
4h
P.67
Osteoporosis 4h
Cont
15
30
4h
P.68
Consol. Delay 8h
Cont
12
20
8h
P.69
Pseudoarthros.8h
Cont
12
20
8h

Rev. 01 del
25/06/14
13/21
P.70
Osteoporosis 8h
Cont
15
20
8h
P.71
Acute pain
Cont
100
50
30
P.72
Chronic pain
Cont
80
50
60
Ca p.5 –APPLICATIONS
See in the attached photos some examples of possible applications with the
solenoids and with carpet, pillow and band (not included); read carefully the
contraindications before using the therapy.
–Therapy planning
Apply the magnetic therapy following your doctor’s indications. Generally
magnetotherapy is used every day and, if necessary, 2 or 3 sessions a day after some
hours. At the end of the programmed therapy if the problem is still present talk to
the doctor.
–Intensity regulation
The intensity can be increased, during the program, to produce more effects or
decreased in case of overheating or feeling of pain.
NOTE: if the intensity or its regulation cause elevated heating or pain in the treated
zone, it is necessary to reduce immediately the intensity of stimulation or eventually
to interrupt the application.
Recommended intensities for most common applications:
Zone of application
Intensity adviced
Bones
50 G
Muscles
40-50 G
Tendons / Ligaments
30-40 G
Joints / Cervical / Lumbago
30-50 G
Prolonged applications (+ di 60 minutes)
20-30 G
–Body position during the sessions
The ideal position of the body during the sessions is relaxed and supine or prone
according to the application zone. The position must be maintained during all the
session to facilitate the effects produced by the magnetic field, particularly the
increased local blood flow, consequence of the induced heating in the treated zone.

Rev. 01 del
25/06/14
14/21
Ca p.6 –POWER SUPPLY
MAGNETER PRO must be supplied by power mains with the provided cable.
For the correct identification of power supply cable furnished in endowment of
base, consult the Chap. "Technical characteristics". Neither the feeder, neither the
battery must be replaced from personal not experienced and with different devices
from those furnished from the house builder.
Ca p.7 –SYMBOLS
CLASSE II DEVICE
BF TYPE DEVICE
ATTENTION, CONSULT THE ATTACHED DOCUMENTATION
THIS DEVICE IS CE MARKED ON CEE NORM 93/42 No. 0476.
0476
Ca p.8 –MAINTENANCE
–Solenoids, band, cushion and carpet
The cable of connection must periodically be checked for verifying that there is no
cracks, possible cause of the dispersion of the magnetic fields,; clean periodically
solenoids, cushion and carpet and with a damp cloth.
–Device and power supply cable
To clean power supply cable and instrument, use a damp cloth. Don't use in any case
liquids, because these devices are not protected from their entry (IP20).
–Feeder substitution
Check the state of the isolations of the power supply cable, before connecting it. If
it’s damaged, also only partially, immediately replace it.

Rev. 01 del
25/06/14
15/21
–Immediate maintenance
Immediate maintenance must be executed by New Age Italia or authorized
personnel if one of the following situations happens:
external mechanical solicitations (ex. serious falls);
strong overheating (ex. if left next to sources of intense heat);
some liquids can be penetrated inside the device;
the feeder, the wrap or other parts of the instrument are damaged, broken or
lacking;
the functionality of the instrument appears altered.
To the goals of the safety it recommends him not to operate with accessories
(es. you manipulate and feeder) different from the furnished ones as endowment of
base.
The frequency of maintenance, of functional control and verification of
correspondence to the safety norms EN60601-1 for the medical devices, to perform
him with secur-tester, it is annual. The useful life of the tool is guaranteed only
from the firm if such maintenance is regularly effected.
NOTE WELL: it is recommended to make the controls be performed only by New
Age Italia; the device can be sent directly to the laboratories of assistance or
delivered to the retailer where it has been purchased.
Assistance center:
New Age Italia srl - Via De Brozzi, 3 - 48022 Lugo (RA)
Ca p.9 –WARNINGS
Do particular attention in the employment of the solenoids not to compromise the
effectiveness of the treatment.
Use the instrument only with electric structures conforming to the actual safety
Norms.
The instrument has degree of protection IP20 (see Chap. "Technical features")
and do not use it close to liquids, because it's not protected from their entry.
Do not use close to cellular telephones (maintain at least one meter of distance).
To operate close (less than 1 meter) to an instrument with short waves therapy or
microwaves, because it can produce instability in the emission.
Don't simultaneously connect the patient with the MAGNETER PRO and with a
surgical instrument HF, to avoid dangers for the patient and for the instrument.
The device works according to its specifications if the environment is maintained
to a temperature between the 5° and the 40° C and with damp inferior to the
80%. The same conditions must be maintained during the transport and the store.

Rev. 01 del
25/06/14
16/21
In case of malfunctions and breakdowns, it is opportune to exclusively send the
device to the house builder.
It's recommended not to operate in proximity of inflammable substances.
Don't use gel and different accessories from those furnished in endowment.
It's important to inform the patient on the type of feeling to perceive during the
therapy, to immediately intervene or interrupt the session switching off the
device or removing the solenoids, in case of different or uncorrect perceptions.
If the power or its regulation cause elevated heating or pain in the treated zone it
is necessary to immediately reduce the intensity of stimulation or eventually
interrupt the application.
Keep away from children.

Rev. 01 del
25/06/14
17/21
Ca p.10 –TECHNICAL FEATURES
–Power supply
Power supply: power mains PRI: 230V50 Hz
–Output features
Max power intensity (P): 200 Gauss for solenoid
300 Gauss for pillow
300 Gauss for band
400 Gauss for the mattress
Frequency (f): 1-100 Hz
Frequency modulation: continue
Modulation wave form: rectangular
–Other features
Dimensions: 225x170x120h [mm]
Weight: about 1 [Kg]
Class: I Tipo: BF
Classification to liquids entry: IP20
Safety in presence of inflammable anaesthetic gas: it’s not of AP or APG
cathegory
Device for working: continuous
Built according to the rules:
EN 60601-1 (1998) –Electromedical devices: Safety General rules
EN 60601-1-2 (1998) –Collateral norm: Electromagnetic compatibility -
Prescriptions and tests
EN 60601-1-4 (1997): Collateral rule: electromedical programmable systems
EN 60601-2-5 (2001) –Electromedical devices: particular rules for the safety of
the equipments of magnetotherapy
IEC 601-2-10 (1987) –Electromedical devices: particular rules for the safety of
neuromuscular stimulators.
CEI 62-84 (1997) –Symbols for electromedical devices
EN60601-1-1 (2002): Collateral rule: Safety prescriptions for electromedical
systems.
Serial number (S.N)
Magneter PRO identity code
S.N. MPRO ZZ XXXX
Device identity number
Year of production

Rev. 01 del
25/06/14
18/21
Ca p.11 –ENDOWMENT OF BASE / ACCESSORIES
–Endowment of base
Device
Power supply cable
N.1 rubber solenoids couple
N.2 elastic bands
Instructions
–Accessories and consumed materials
Rubber solenoids couple
Elastic bands
Band solenoid with welcro cm 91,5x20,5 (open)
Cushion solenoid cm 45x45
Carpet solenoid cm160x60
Ca p.12 –BIBLIOGRAPHY
C. Menarini, M. Menarini: Manuale di terapia fisica, Aulo Gaggi Editore,
Bologna 1985
M. Moselli, M. Manca: Fisioterapia pratica, Ed. Minerva Medica, Torino 1993
B. Gialanella, G. D’alessandro, R. Santoro: Terapia fisica pratica, ED.
Marrapese, Roma 1997
Vasta: Manuale pratico illustrato di terapia fisica, ED. Marrapese, Roma
1998
Cisari, G. Severini: Fisioterapia clinica pratica, Edi-ermes, Milano 1999
T.Thorossian: Magnetic field therapy, Ed. NeoMedica, Vienna 1999
G. Nanni, G. S. Roi, D. Vasapollo: Le lesioni muscolari dell’arto inferiore
nello sportivo, ED. Marrapese, Roma 2000

Rev. 01 del
25/06/14
19/21
DECLARATION OF CONFORMITY
The undersigned New Age Italia s.r.l.
Via De Brozzi,3 –48022 Lugo (RA)
Tel: +39 0545 32019 –Fax +39-0545 369028
in the person of its legal representative, Mr. Barnabè Manuel declares that
the following class IIa device:
Brand Name: New Age Italia
Model: Magneter PRO
is a MADE IN ITALY product, made by New Age Italia s.r.l.;
is up-to the requisites prescribed in EEC Directive 93/42CEE modified by Directive 2007/47CE
concerning health regulations and absorbed by Legislative Decree no. 37 dated 25/01/2010;
that the procedure for the evaluation of conformity described in Annex II to the above-
mentioned Directive has been duly performed;
and that the following harmonized standards have been applied:
EN 60601-1: 2007
EN 60601-1-2: 2003
EN 60601-1-4: 1997
EN 980-2003
EN 1041:1998
The notified authority no. 0476 has, in virtue of the certificate MED26017, issued a permission
to apply a CE conformity mark to the above-mentioned regulations prescribed in EEC
Directive 93/42CEE and modified by Directive 2007/47CE absorbed by Legislative Decree no.
37 dated 25/01/2010.
Lugo, 03/11/2010
The company’s legal representative
Table of contents
Other New Age Medical Equipment manuals

New Age
New Age HI-E Sonyc User manual

New Age
New Age BIOSONYC User manual

New Age
New Age iTens Terapix User manual

New Age
New Age LASETRON CARD User manual

New Age
New Age TEKRA CT User manual

New Age
New Age Tekra life User manual

New Age
New Age FARMA BIKE User manual

New Age
New Age MAGNETER POCKET User manual

New Age
New Age Hi-Laser Beta LASER User manual

New Age
New Age MAGNETO BIOLIFE User manual