2010-8401CE Rev C
6May 2011
MARK 5 Nuvo LITE Serial No. ______________
Date rst used: ___________________________
Maintained by: ___________________________
_________________________________________
Your distributor: _________________________
Address : ________________________________
_________________________________________
Telephone : ______________________________
PREVENTIVE MAINTENANCE:
a. Wash cabinet lter weekly.
b. Inspect inlet air lter at each patient visit.
Replace lter every 2 years, or more often
depending on environment.
c. Check oxygen concentration every 15,000 hours or
3 years to verify the continuing OCSI function.
The manufacturer’s instructions for the preventive
maintenance of the devices are de ned in the
maintenance manual. Check with your service
provider for any updates to recommended schedules.
The work must be carried out by suitably trained
technicians certi ed by the manufacturer.
Use original spare parts only (see Pg. 7).
Uponrequest,thesuppliercanprovidecircuitdiagrams,
sparepartslists,technicaldetailsoranyotherinformation
of use to quali ed technical personnel for parts of the
devicewhicharedesignatedasbeingthemanufacturer’s
responsibility or by the manufacturer as repairable.
Medical Device Regulations require users and
service providers to report to the manufacturer any
incident that could, if repeated, result in injury to
any person.
IV.8. Method for disposing of waste
AllwastefromtheMARK5NuvoLITE (patient circuit, lter,
etc.) must be disposed of using the methods appropriate to
the civil authority of the location where disposed.
IV.9. Method for disposing of the device
In order to preserve the environment, the concentrator must
only be disposed of using the appropriate methods. All ma-
terials of construction are recycleable.
Furthermore, as part of the marking (directive 93/42/EEC),
the serial number of the device disposed of must be sent to
the Nidek Medical technical service department if the unit
has the marking.
Accuracy of ow supplied:
In compliance with the ISO 8359 standard, the ow supplied
is equal to the ow set on the owvalve, accurate to within ±
10 % or 200 ml/min, whichever is the larger of the two.
Oxygen Concentration:
• at 2 l/min: >90%. .
• at 5 l/min: 90%. (+6.5%/-3%)
(Values at 21oC and at one atmosphere pressure).
Maximum recommended ow: 5 l/min.
The variation of the maximum recommended ow does not
exceed ± 10 % of the indicated value when a back pressure
of 7 kPa (1 psig) is applied to the output of the device. The
maximum outlet pressure is 50 kPa (7 psig).
Electrical power supply:
115 V Units 230 V Units
Frequency: 60Hz 50 & 60Hz
Average Power: 330W(avg) 300 W(avg)
Protection Class: Class II Class II
Mains Protection: 10A 5A
Filters:
At the rear of the device: a cabinet air lter.
At the compressor input: an inlet air lter, 5 µm, located
behind the cabinet air lter.
Before the oxygen outlet: a nal product lter, < 0.3 µm.
(technician only).
Air circulation:
A tubeaxial fan cools the compressor compartment.
Environmental limit conditions:
The performances of the device (especially the oxygen
concentration)arequotedat21oC(70oF)andoneatmosphere.
They may change with temperature and altitude. For further
information, please consult the maintenance manual.
• The device must be stored, transported and used in the
vertical position only.
• Ambienttemperatureofbetween5oCand 40oC(40oFto
104oF) operation.
• Storage temperature from -20oC to 60oC (-4oF to 160oF).
• Relative humidity of between 15 % and 95 % operation
and storage, both non-condensing.
• Altitude(21oC): Up to 2,286m (7,500ft) without degra
dation; Consult your equipment provider for further in
formation regarding 2,286m to 4000m (7,500 to
13000ft).
• Complies with EN60601-1 standard; spilling of a glass
of water.
IV. 7. Standards
ISO 8359:1996 Oxygen concentrators for medical use.
EN60601-1[UL60601-1:2003],CAN/CSA-C22.2No.601.1-
M90 w/A1&A2: Electrical Safety- Medical Devices.
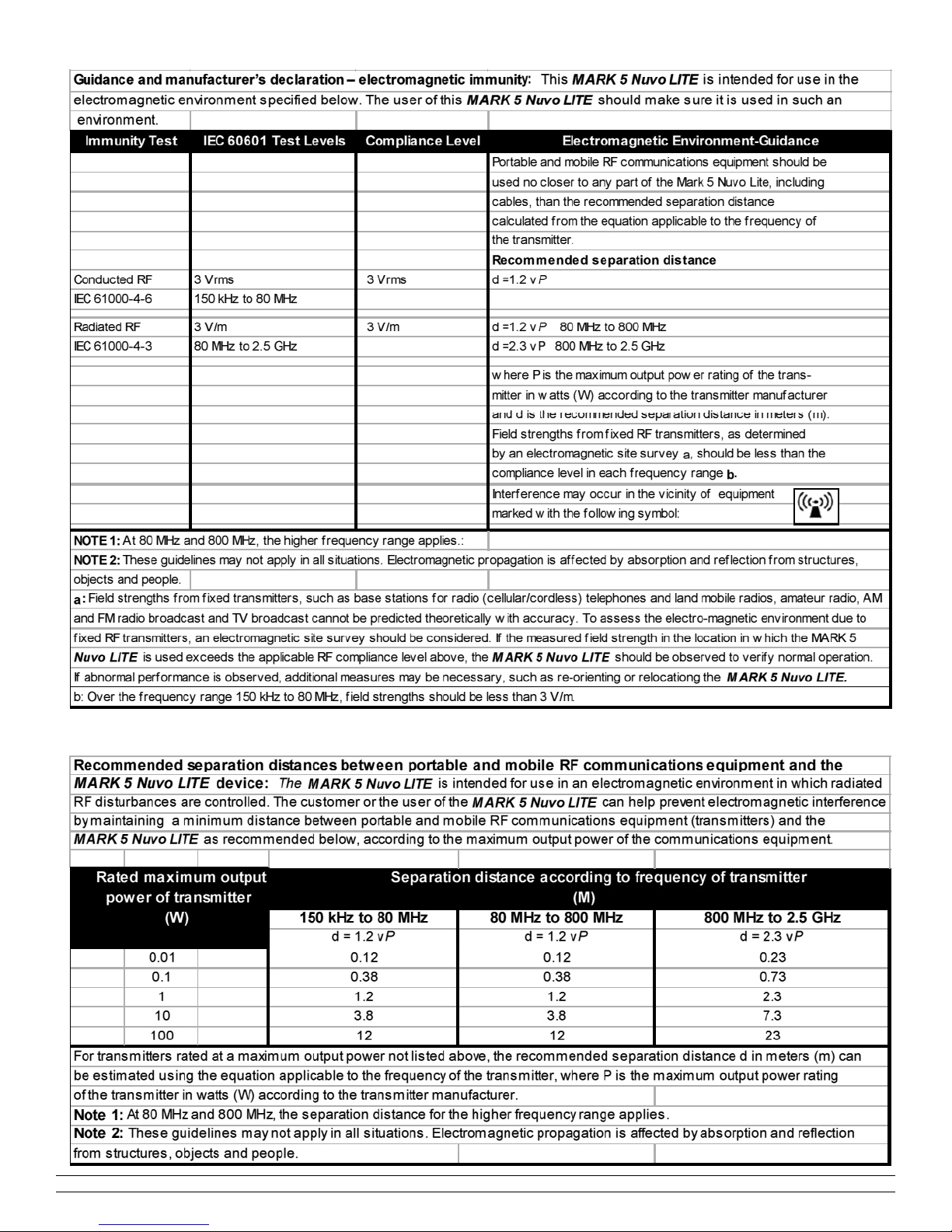
EN60601-1-2:2001 Electromagnetic Compatibility