2010-2238CE Rev C
6May 2011
Nuvo Serial No. ___________________________
Date rst used: ___________________________
_________________________________________
Maintained by: ___________________________
_________________________________________
Your distributor: _________________________
Address : ________________________________
_________________________________________
_________________________________________
Telephone : ______________________________
PREVENTIVE MAINTENANCE:
a. Wash cabinet lter weekly.
b. Inspect inlet air lter at each patient visit.
Replace lter annually, or more often depending on
environment.
c. Check oxygen concentration every 15,000 hours or
3 years to verify the continuing OCSI function.
The manufacturer’s instructions for the preventive
maintenance of the devices are de ned in the
maintenance manual. Check with your service
provider for any updates to recommended schedules.
The work must be carried out by suitably trained tech-
nicians certi ed by the manufacturer.
Use original spare parts only (see Pg. 7).
Uponrequest,thesuppliercanprovidecircuitdiagrams,
sparepartslists,technicaldetailsoranyotherinformation
of use to quali ed technical personnel for parts of the
devicewhicharedesignatedasbeingthemanufacturer’s
responsibility or by the manufacturer as repairable.
Medical Device Regulations require users and
service providers to report to the manufacturer any
incident that could, if repeated, result in injury to
any person.
IV.8. Method for disposing of waste
AllwastefromtheMark5Nuvo8 (patient circuit, lter, etc.)
mustbedisposedofusingthemethodsappropriatetothecivil
authority of the location where disposed.
IV.9. Method for disposing of the device
In order to preserve the environment, the concentrator must
only be disposed of using the appropriate methods. All ma-
terials of construction are recycleable.
Furthermore, as part of the marking (directive 93/42/EEC),
the serial number of the device disposed of must be sent to
the Nidek Medical technical service department if the unit
has the marking.
Accuracy of ow supplied:
In compliance with the ISO 8359 standard, the ow supplied
is equal to the ow set on the owmeter, accurate to within ±
10% or 200 ml/min, whichever is the larger of the two.
Average oxygen content:
8 l/min: 90%. +5.5% / -3.0%
(Values at 21oC and at one atmosphere pressure).
Minimum recommended ow, 2 lpm.
Maximum recommended ow, 8 lpm.
The variation of the maximum recommended ow does not
exceed ± 10 % of the indicated value when a back pressure
of 6.9 kPa (1 psig) is applied to the output of the device. The
maximum outlet pressure is 117 kPa (17 psig).
Electrical power supply:
115 V Units 230 V Units
Frequency: 60Hz 50/60Hz
Average Power: 490 watts 490/585 watts
Protection Class: Class II Class II
Mains Protection: 10A 5A
Filters:
At the rear of the device: a cabinet air lter.
At the compressor input: an inlet air lter, behind cabinet
air lter.
Before the oxygen outlet: a nal product lter < 0.3 µm.
(technician only)
Air circulation:
One tubeaxial fan cools the compressor compartment and a
second fan cools the heat exchanger coil.
.
Environmental limit conditions:
Theperformanceofthedevice(especiallytheoxygenconcen-
tration) are quoted at 21oC (70oF) and one atmosphere. They
may change with temperature and altitude. For further infor-
mation, please consult the maintenance manual.
• The device must be stored, transported and used in the
vertical position only.
• Ambient temperature of between 10oC and 40oC (50oF
to 105oF) operation.
• Storage temperature from -20oC to 60oC (0oF to 140oF).
• Relative humidity of between 15 % and 95 % operation
and storage, both non-condensing.
• Altitude(21oC): Up to 1500m (5000ft) without degrada-
tion; Consult your equipment provider for further infor
mation regarding 1500 m to 4000m (5000 to 13000ft).
• Complies with EN60601-1 standard; spilling of a glass
of water.
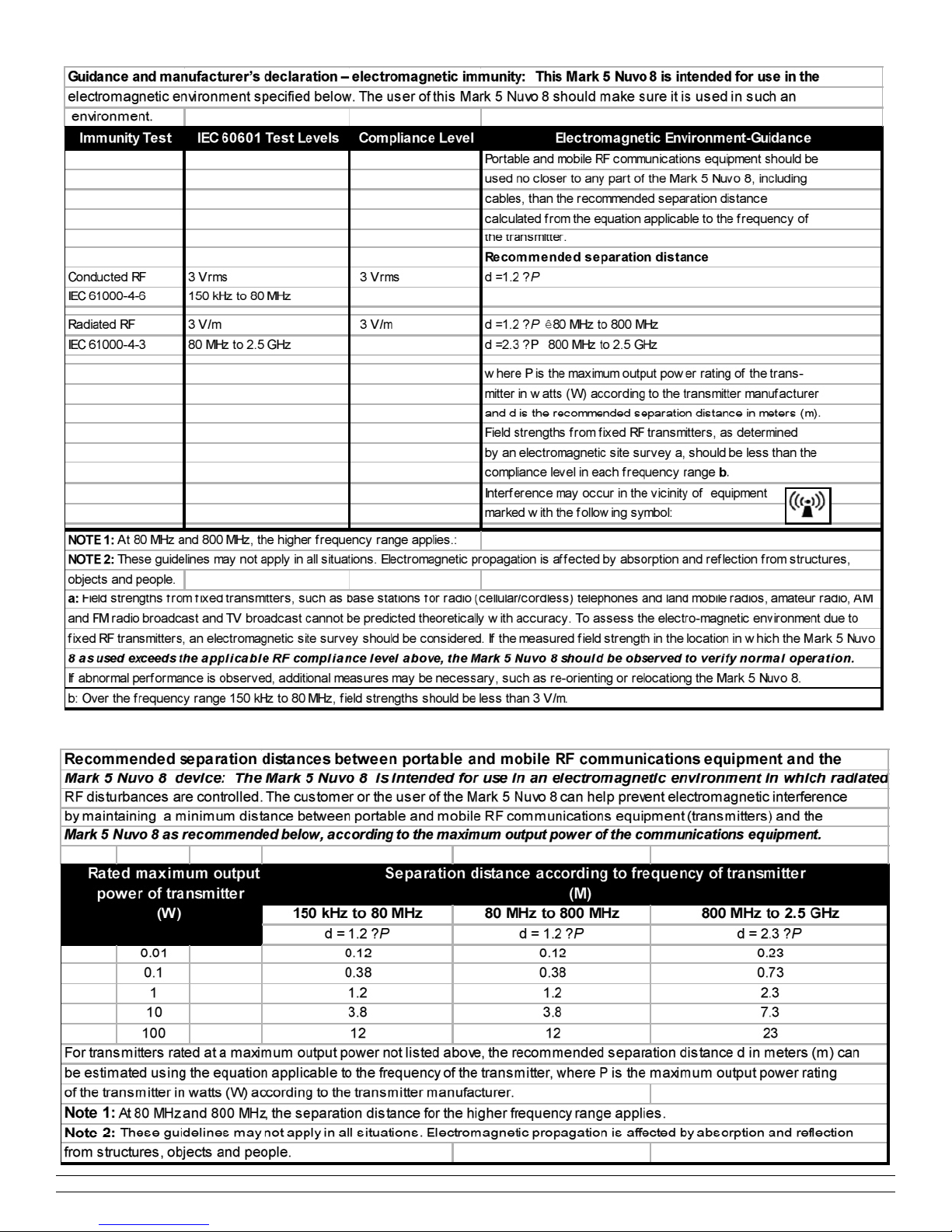
IV. 7. Standards
ISO 8359:1996 Oxygen concentrators for medical use.
EN60601-1[UL60601-1:2003],CAN/CSA-C22.2No.601.1-
M90 w/A1&A2: Electrical Safety- Medical Devices.
EN60601-1-2:2000 Electromagnetic Compatibility