______________________________________________________________________________________________________________
2010-2324CE-G March 2022 Page 3 of 8
EN
Use the power cord provided.
Check that the electrical characteristics of the power
outlet used match those indicated on the manufacturer’s
plate on the rear panel of the device.
This unit may be equipped with a polarized plug. That is
one blade wider than the other. If it does not fit into the
outlet, reverse the plug. If it still does not fit, contact a
qualified electrician. Do not defeat this safety feature.
2.3 Alarms and Safety Features
The device has an audible alarm to warn the user of
problems. In order that the alarm may be heard, the
maximum distance that the user can move away from it
must be determined to suit the surrounding noise level.
No voltage detection: In the event of a loss of mains
power, an intermittent audible alarm is activated and the
green light turns off.
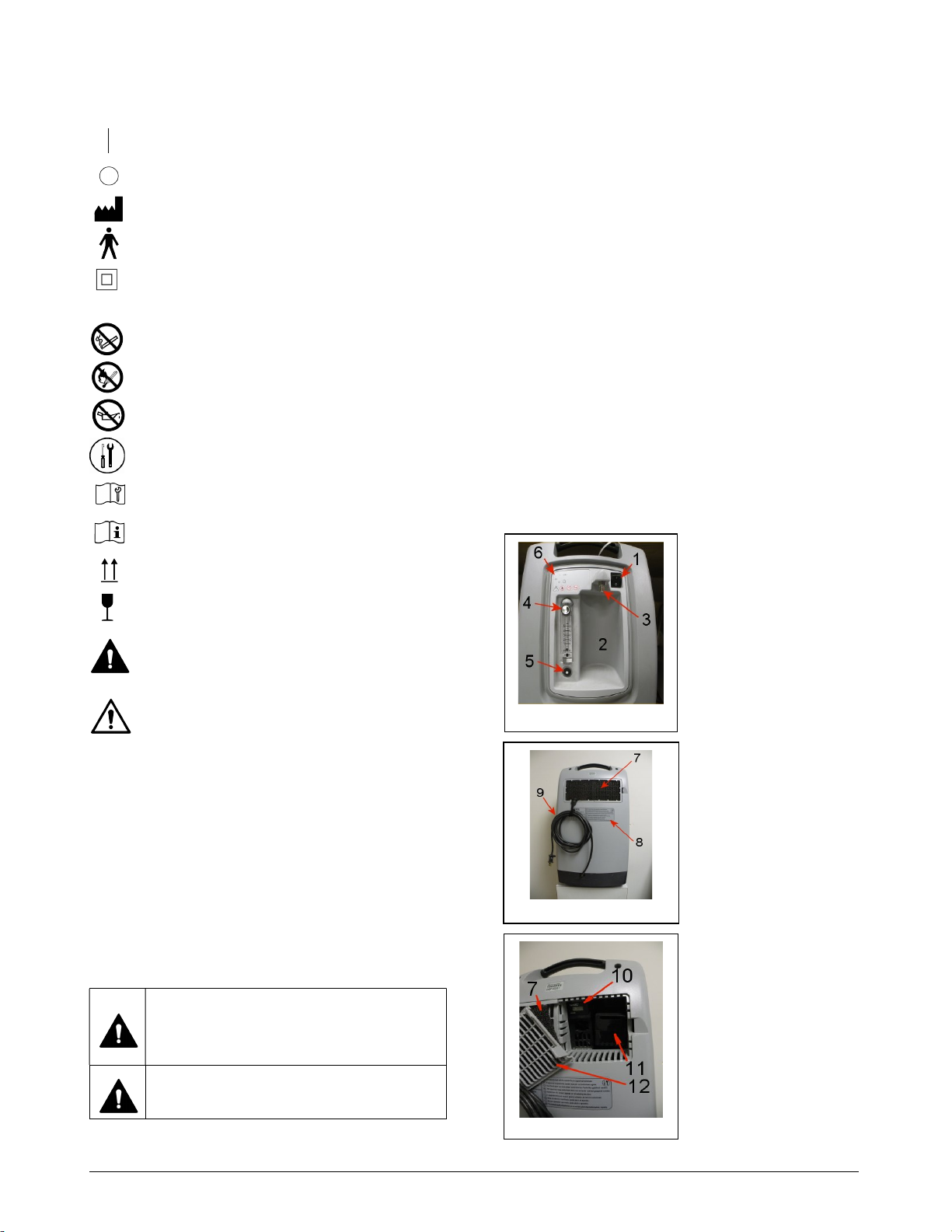
Test alarm by actuating the Power Switch (Fig 1-1) when
the mains cable is not plugged into the power outlet.
Oxygen Concentration Status Indicator: The oxygen
concentration monitor is an electronic module capable of
checking the effective oxygen concentration supplied by
the concentrator. The oxygen monitor measures the
concentration and activates an audible and visual alarm if
it falls below the alarm set point percentage. When the
device is started, the indicator lights (Fig 2-6) located on
the front panel operate as described below.
Green indicator: This light indicates that power is
applied to the concentrator and that it is ready to provide
oxygen enriched air to the patient.
On initial power up, this indicator light will flash green
until the device has reached normal operating conditions.
This should happen within approximately 2 minutes.
Yellow indicator: This light and a continuous audible
alarm will activate when the oxygen concentration level
falls below the set point.
Devices manufactured prior to 2018 included a red indicator light.
No special maintenance is required. The alarm set-point is
factory set and the setting cannot be adjusted.
All OCSI models are set at 85% ± 3%.
Blocked Cannula detection: If supplied, the device has a
Blockage Alarm. A continuous audible alarm and both
indicator lights will be lit immediately in the event the
flow of oxygen to patient becomes blocked.
Malfunction detection: If low pressure occurs due to a
mechanical failure, the indicator light will flash yellow
and a continuous audible alarm will actuate.
If any of the above alarm conditions occur, press the
Power Switch (Fig 1-1) to the “O” (OFF) position.
Call your equipment supplier to service the device.
Thermal safety: The compressor motor is protected by a
thermal switch situated in the stator winding (145 ± 5° C).
One tubeaxial fan cools the compressor compartment and
additional fans cool the heat exchanger coil for the Nuvo
8 and Nuvo 10 models (585, 985, 595, and 1005).
Electrical protection:
A 5A circuit breaker is incorporated into the front
cabinet of all 230V models
A 10A circuit breaker is incorporated into the front
cabinet of all 115V models
Class II devices with insulated casings (EN60601-1 standard)
Safety valve: This is fitted on the compressor outlet and
is calibrated to 3.4 bar (50 psig).
Fire Break: This device is fitted with a metal fire break
at the Oxygen Product Outlet (Fig 1-3). This break will
keep fire from entering the device. See “Accessories and
Spare Parts” (§ 2.5) for fire safe accessories.
2.4 Device Performance and Specifications
The performance of the device (especially the oxygen
concentration) is quoted at 21°C (70°F) and one
atmosphere. The specifications may change with
temperature and altitude.
Model 505/565 905/965 585 985 595 1005
Description 5lpm
115V
5LPM
230V
8LPM
115V
8LPM
230V
10LPM
115V
10LPM
230V
Frequency 60Hz 50/60Hz 60Hz 50/60Hz 60Hz 50Hz
Average
Power
410
Watts
420
Watts
500
Watts
490
Watts
700
Watts
600
Watts
Protection
Class Class II
Mains
Protection 10A 5A 10A 5A 10A 5A
Average
Oxygen
Content
At 2 LPM
> 90%
At 2 LPM
> 90%
At 2 LPM
> 90%
Average
Oxygen
Content
At 5 LPM
87% to 95.5%
At 8 LPM
87% to 95.5%
At 10 LPM
87% to 95.5%
Liter Flow 1 to 5 LPM 2 to 8 LPM 2 to 10 LPM
Outlet
Pressure 7 Psig 15 Psig 20 Psig
Dimensions
(L x W x H) 394 x 396 x 706 mm (15.5 x 15.6 x 27.8 in.)
Weight 24.5-26 kg (54-58 lbs)*
Noise Level < 58 dBA
* Weight dependent on model and features
In compliance with EN ISO 80601-2-69, the flow supplied is
equal to the flow set on the flowmeter, accurate to within
± 10% or 200 ml/min, whichever is greater.
The variation of the maximum recommended flow does not
exceed ± 10 % of the indicated value when a back pressure
of 6.9 kPa (1 psig) is applied to the output of the device.
Materials in direct or indirect contact with the patient
Concentrator enclosure ABS/Polycarbonate
Printed labels Polycarbonate
Power switch (Fig 1-1) Nylon
Oxygen product outlet (Fig 1-3) SS or Brass
Flow adjustment knob (Fig 1-4) ABS