ENG – Translations of the IFU can be found using this QR code.
FRE – Les traductions de cette notice d’utilisation peuvent être retrouvées à l’aide de ce code QR.
GER – Übersetzungen dieses Handbuchs können über diesen QR-Code abgerufen werden.
ITL – Utilizzando questo codice QR, è possibile trovare le traduzioni delle Istruzioni per l’uso.
SPA – Las traducciones de este manual se pueden encontrar utilizando este código QR.
POR – Pode aceder às traduções das instruções de utilização através deste código QR.
DUT – Vertalingen van de handleiding zijn te vinden met behulp van deze QR-code.
GRK – ΜετηχρήσηαυτούτουκωδικούQRμπορείτεναβρείτεμεταφράσεις
τωνοδηγιώνχρήσης(IFU).
DAN – ScandenneQR-kodeforatndeoversættelserafdennebrugsvejledning.
SWE – Översättningar av den här guiden kan hittas med denna QR-kod.
FIN – Käyttöohjeen käännökset löytyvät tällä QR-koodilla.
POL – TłumaczeniategoprzewodnikamożnaznaleźćzapomocątegokoduQR.
NOR – OversettelseravdennebruksanvisningenkannnesvedåbrukedenneQR-koden.
Instructions for Use / Operator’s Manual
https://www.nonin.com/support/2500
P/N 114856-001-01 11/2022 13700 1st Avenue North, Plymouth, MN 55441-5443 USA nonin.com
Instructions for Use
Model 2500
©2022 Nonin Medical, Inc. All trademarks are the property of Nonin Medical, Inc. unless otherwise noted.
Cautions (continued)
• This equipment complies with IEC 60601-1-2 for electromagnetic compatibility for medical electrical equipment and/or systems. This
standard is designed to provide reasonable protection against harmful interference in a typical medical installation. However, because of the
proliferation of radio-frequency transmitting equipment and other sources of electrical noise in healthcare and other environments, it is possible
that high levels of such interference due to close proximity or strength of a source might disrupt the performance of this device. Medical electri-
cal equipment needs special precautions regarding EMC, and all equipment must be installed and put into service according to the
EMCinformationspecied.
• IncompliancewiththeEuropeanDirectiveonWasteElectricalandElectronicEquipment(WEEE)2002/96/EC,donotdisposeofthisproductas
unsorted municipal waste. This device contains WEEE materials; please contact your distributor regarding take-back or recycling of the device.
If you are unsure how to reach your distributor, please call Nonin for your distributor’s contact information.
• This device’s display will go blank after 10 seconds of inadequate signals. The data update period is every 1.5 seconds.
• This device is designed to determine the percentage of arterial oxygen saturation of functional hemoglobin. Factors that may degrade pulse
oximeter performance or affect the accuracy of the measurement include the following:
• A functional tester cannot be used to assess the accuracy of a pulse oximeter monitor or sensor.
• AllpartsandaccessoriesconnectedtotheserialportofthisdevicemustbecertiedaccordingtoatleastIEC60950orUL1950for
data-processing equipment.
• This device is a precision electronic instrument and must be repaired by trained Nonin personnel only. Field repair of the device is not possible.
Do not attempt to open the case or repair the electronics. Opening the case may damage the device and void the warranty.
• Anysignorevidenceofopeningthesystem,eldservicebynon-Noninpersonnel,tampering,oranykindofmisuseorabuseofthesystem,shall
void the warranty in its entirety.
• Replacebatterieswithin30secondstoavoidlosingsettings(date,time,andpatientdatastoredinmemory)orcorruptingdata.
• Radiosandcellphonesorsimilardevicescanaffecttheequipmentandmustbekeptatleast2meters(6.5feet)awayfromequipment.
• Failureofanetworkdatacoupling(serialcable/connectors/wirelessconnections)willresultinlossofdatatransfer.
Cautions – only for the Model 2500A PalmSAT Pulse Oximeter with Alarms
• Setting alarm limits to extremes can render the alarm system useless.
•Excessive ambient light •Moisture in the sensor •Cardiogreen and other intravascular dyes
•Excessive motion •Improperly applied sensor •Carboxyhemoglobin
•Electrosurgical interference •Incorrect sensor type •Methemoglobin
• Bloodowrestrictors(arterialcatheters,
blood pressure - carboxyhemoglobin cuffs,
infusionlines,etc.)
•Inadequate signal •Dysfunctional hemoglobin
•Venous pulsations •Articialnailsorngernailpolish
•Anemia or low hemoglobin concentrations •A sensor not at heart level
Nonin Model 2500C Charger Stand
Indications for Use/Intended Use/Intended Purpose
The Nonin Model 2500C Charger Stand is intended for use with the PalmSAT Models 2500 and 2500A Pulse Oximeters and the Model 2500B
RechargeableNiMH(NickelMetalHydride)BatteryPack.
Warnings
• Do not use this product in an MR environment.
• Do not use this product in an explosive atmosphere.
• ThisproductisnotdebrillationproofperIEC60601-1.
• As with all medical equipment, carefully route patient cabling to reduce the possibility of patient entanglement, strangulation, or injury to
the patient.
• This product should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the product should be
observed carefully to verify normal operation.
• The use of accessories, sensors, cables, and power supplies other than those listed in the Parts and Accessories List may result in increased
electromagnetic emission and/or decreased immunity of this product.
• Topreventimproperperformanceand/orpatientinjury,verifycompatibilityofthemonitor,sensor(s),andaccessoriesbeforeuse.
• Nomodicationstothisdeviceareallowedasitmayaffectdeviceperformance.
• PortableRFcommunicationsequipmentsuchascellphonesorradios(includingperipheralssuchasantennacablesandexternalantennas)
shouldbeusednocloserthan30cm(12inches)toanypartoftheMEsystem,includingcablesspeciedbythemanufacturer.Otherwise,
degradation of the performance of this equipment could result.
Cautions
• This equipment complies with International Standard 60601-1-2 for electromagnetic compatibility for medical electrical equipment and/or
systems. This standard is designed to provide reasonable protection against harmful interference in a typical medical installation. However,
because of the proliferation of radio-frequency transmitting equipment and other sources of electrical noise in healthcare and other
environments, it is possible that high levels of such interference due to close proximity or strength of a source might disrupt the performance
of this product. Medical electrical equipment needs special precautions regarding EMC, and all equipment must be installed and put into
serviceaccordingtotheEMCinformationspeciedinthismanual.
• Do not connect this product to an AC outlet controlled by a wall switch. If the switch is accidentally turned off before the battery pack is
recharged, the pulse oximeter may not function.
• Grounding reliability can only be achieved when the equipment is connected to an equivalent receptacle marked “Hospital Only” or “Hospital Grade”.
• This product contains sensitive electronic components and must be repaired by trained Nonin personnel only.
• Do not immerse this product in liquid.
• Do not place liquids on top of this product.
• Do not use caustic or abrasive cleaning agents on this product.
• Do not remove any covers from the product. There are no user-serviceable parts inside the unit.
• Do not attempt to charge disposable batteries. Disposable batteries may leak or explode if used improperly.
• Follow local, state and national governing ordinances and recycling instructions regarding disposal or recycling of the product and product
components, including batteries. Use only Nonin-approved battery packs.
• IncompliancewiththeEuropeanDirectiveonWasteElectricalandElectronicEquipment(WEEE)2002/96/EC,donotdisposeofthisproduct
as unsorted municipal waste. This product contains WEEE materials; please contact your distributor regarding take-back or recycling of the
product. If you are unsure how to reach your distributor, please call Nonin for your distributor’s contact information.
Adverse Event Statement
Users and/or patients should report adverse events involve their Nonin device to Nonin Medical, Inc. and the competent authority of the EU Member
State in which the user and/or patient is established, if applicable.
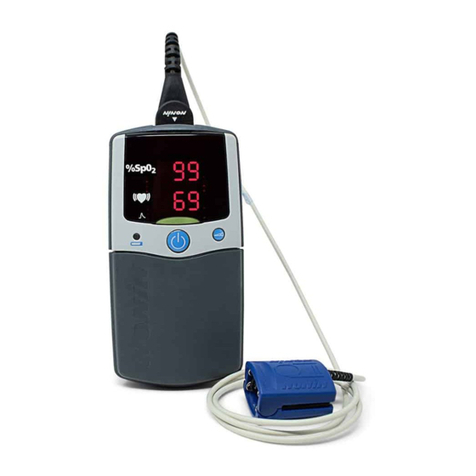
Nonin Model 2500 PalmSAT®Pulse Oximeter and Model 2500A PalmSAT®Pulse Oximeter with Alarms
Indications for Use/Intended Use/Intended Purpose
The Nonin®Model 2500 PalmSAT®Pulse Oximeter and Model 2500A PalmSAT®Pulse Oximeter with Alarms are indicated for use in measuring
anddisplayingfunctionaloxygensaturationofarterialhemoglobin(SpO2)andpulserateforadult,pediatric,andneonatalpatients.Thesedevices
are intended for continuous monitoring and/or spot- checking of patients during both motion and no-motion conditions, and for patients who are
well or poorly perfused.
Warnings
• Do not use this device in an MR environment.
• Explosion Hazard:Donotuseinanexplosiveatmosphereorinthepresenceofammableanestheticsorgasses.
• ThisdeviceisnotdebrillationproofperIEC60601-1.
• This device is intended only as an adjunct in patient assessment. It must be used in conjunction with other methods of assessing clinical signs
and symptoms.
• Oximeterreadingsofthisdevicemaybeaffectedbytheuseofanelectrosurgicalunit(ESU).
• Inspect the sensor application site at least every 6 to 8 hours to ensure correct sensor alignment and skin integrity. Patient sensitivity to sensors
and/or double-backed adhesive strips may vary due to medical status or skin condition.
• To avoid patient injury, use only with Nonin-branded PureLight®pulse oximeter sensors. These sensors are manufactured to meet the accuracy
specicationsforNoninPulseOximeters.Usingothermanufacturers’sensorscanresultinimproperpulseoximeterperformance.
• Topreventimproperperformanceand/orpatientinjury,verifycompatibilityofthemonitor,sensor(s),andaccessoriesbeforeuse.
• Nomodicationstothisdeviceareallowedasitmayaffectdeviceperformance.
• Do not use a damaged sensor. If the sensor is damaged in any way, discontinue use immediately and replace the sensor.
• As with all medical equipment, carefully route patient cabling to reduce the possibility of patient entanglement, strangulation, or injury
to the patient.
• This device should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the device should be
observed carefully to verify normal operation.
• Theuseofaccessories,sensors,cables,andpowersuppliesotherthanthosespeciedinthePartsandAccessoriesListmayresultinincreased
electromagnetic emission and/or decreased immunity of this device.
• This device must be able to measure the pulse properly to obtain an accurate SpO2measurement. Verify that nothing is hindering the pulse
measurement before relying on the SpO2measurement.
• Operation of this device below the minimum amplitude of 0.3% modulation may cause inaccurate results.
• Discontinue use of adhesive tape strips if the patient exhibits an allergic reaction to the adhesive material.
• Avoid excessive pressure to the sensor application site as this may cause damage to the skin beneath the sensor.
• When a system fault occurs, the patient will no longer be monitored.
• To comply with relevant product safety standards, ensure that all alarm volumes are set appropriately and are audible in all situations.
Do not cover or otherwise hinder any speaker openings.
• The device turns off after approximately 10 minutes when at low battery capacity.
• Before changing the batteries, make sure the device is off and the sensor is not attached to a digit.
• PortableRFcommunicationsequipmentsuchascellphonesorradios(includingperipheralssuchasantennacablesandexternalantennas)
shouldbeusednocloserthan30cm(12inches)toanypartoftheMEsystem,includingcablesspeciedbythemanufacturer.Otherwise,
degradation of the performance of this equipment could result.
Warnings – only for the Model 2500A PalmSAT Pulse Oximeter with Alarms
• Verify all alarm settings and limits during system startup to ensure that they are set as intended.
• A hazard can exist if different presets are used on multiple 2500A monitors in one care area.
• Because operating environments vary, use caution to ensure that all audible alarms and indicators can be heard. Users must determine the
acceptable audible distance of all alarms.
• Donotplacethisdeviceinanenvironmentwhereitsspeakeropeningmaybecomeblocked;alarmsmaybecomemufedorinaudible.
• Turning off the alarm volume creates a situation that is not compliant with relevant safety standards. The alarm silence indicator is lit solid
when the alarm volume is turned off or set below 45 dBA.
Cautions
• Before use, carefully read the package insert provided with the sensors.
• This device is not an apnea monitor.
• Verifythatallvisibleindicatorsilluminateandthatanaudibleindicatorsoundsduringthestartup(initialization)sequence.Ifanyindicatoris
not lit or the audible indicator does not sound, do not use the device. Contact Nonin Technical Service for assistance.
• Review all limits to ensure they are appropriate for the patient.
• Thepresenceofadebrillatormayinterferewiththeperformanceofthisdevice.
• This device may not work on all patients. If you are unable to achieve stable readings, discontinue use.
• This device has motion tolerant software that minimizes the likelihood of motion artifact being misinterpreted as good pulse quality. In some
circumstances, however, the device may still interpret motion as good pulse quality. Minimize patient motion as much as possible.
• EarClipandReectancesensorsarenotrecommendedforpediatricorneonataluse.Theaccuracyofthesesensorshasnotbeenestablished
for pediatric or neonatal use.
• Do not autoclave or immerse the device or sensors in liquid. Do not expose the device or components to excessive moisture or liquids.
• Do not use caustic or abrasive cleaning agents on the device or the sensors.
• Theoximetersensormightnotworkoncoldextremitiesduetoreducedcirculation.Warmorrubthengertoincreasecirculation,orreposition
the sensor.
• Replace the batteries as soon as possible after a low-battery indication. Always replace the batteries with fully charged batteries.
• UseonlyNonin-speciedbatterytypeswiththisdevice.
• Do not use fully charged and partially charged batteries at the same time. This may cause the batteries to leak.
• Do not remove any covers other than the battery cover when replacing batteries. There are no user-serviceable parts inside other than the
replaceable batteries.
• Follow local, state and national governing ordinances and recycling instructions regarding disposal or recycling of the device and device
components, including batteries.
• Batteries may leak or explode if used or disposed of improperly.
• Remove the batteries if the device will be stored for more than 1 month.
REP
EC
Warranty
The device warranty
is 3 years.
nonin.com/warranty
Symbol
Glossary
nonin.com/symbols
Compliance
This product complies
withISO10993.
Not made from
natural rubber latex.
MPS, Medical Product Service GmbH
Borngasse 20
D-35619Braunfels,Germany
MedEnvoy Switzerland
Gotthardstrasse 28, 6302 Zug
Switzerland
REP
CH
For summary of safety and clinical data see above QR code.