9
2.PREPARING THE SETUP FOR MEASUREMENT
2.1 Starting the system
Powering up the instrument
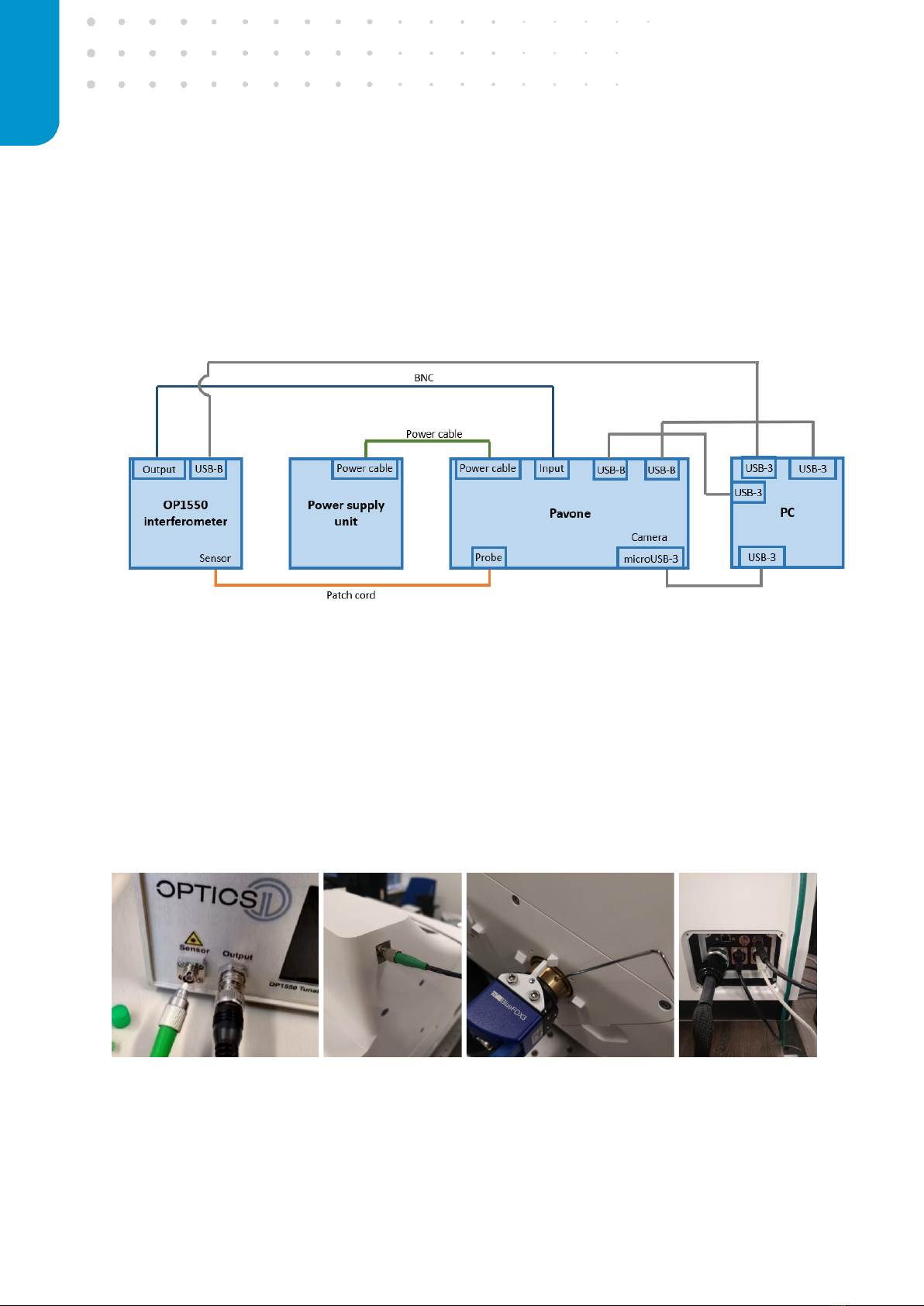
Before starting up the instrument for the first time, make sure that the stage lock is removed (follow the
installation manual provided with the system), all cables are connected correctly and the power switches
on the back of the boxes are turned on. Switch on the devices:
1. Interferometer
2. Power supply
3. PC
The interferometer will show a live measurement signal on the LCD screen after the initialization of the
laser is completed. The power supply unit automatically powers the Pavone unit.
Starting the software
Ensure that stage locks are removed, nothing can obstruct sample stage movement. Start the Pavone
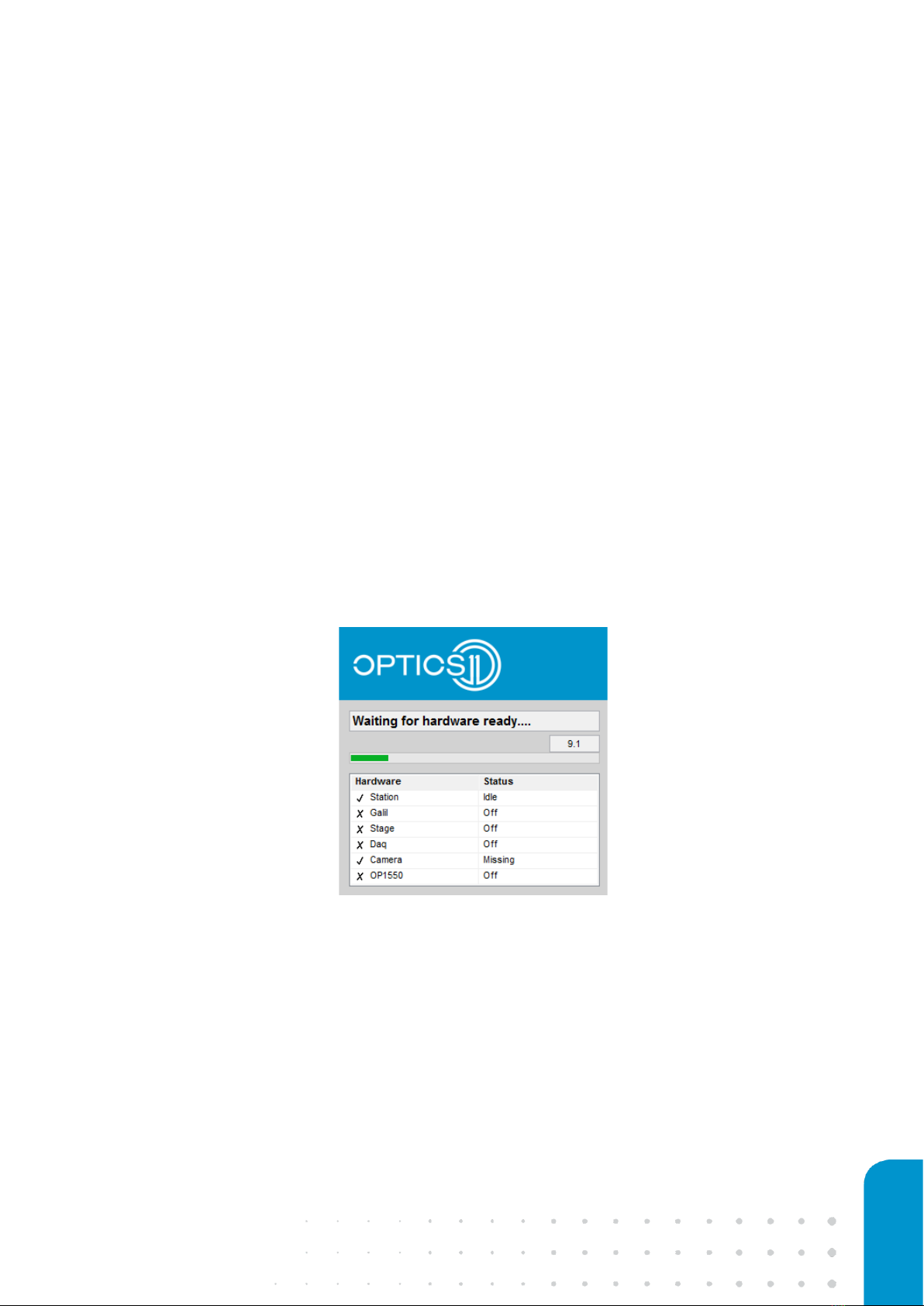
measurement software by double-clicking the Pavone software icon on the desktop. The software is
loading all devices while checking the hardware connection status, which should all be ‘Idle’ (see Figure
5) after a maximum waiting time of 60 seconds. The software will then proceed to the main user interface.
Figure 5: Hardware status check.
After starting the instrument the system will prompt homing of the XY sample stages: this is required to
‘zero’ the absolute position of the sample stages and ensure accurate positioning during use. After
homing the stages the system state will switch to ‘idle’.
2.2 Mounting a probe to the Pavone
Optics11 Life force sensors, also called probes, consist of optical fiber and cantilever (MEMS) that are
glued to glass ferrule perpendicularly. At the end of the cantilever, there is a spherical tip mounted either
directly on the cantilever or through an extended rod (see Figure 6). The spherical tip is used to deform