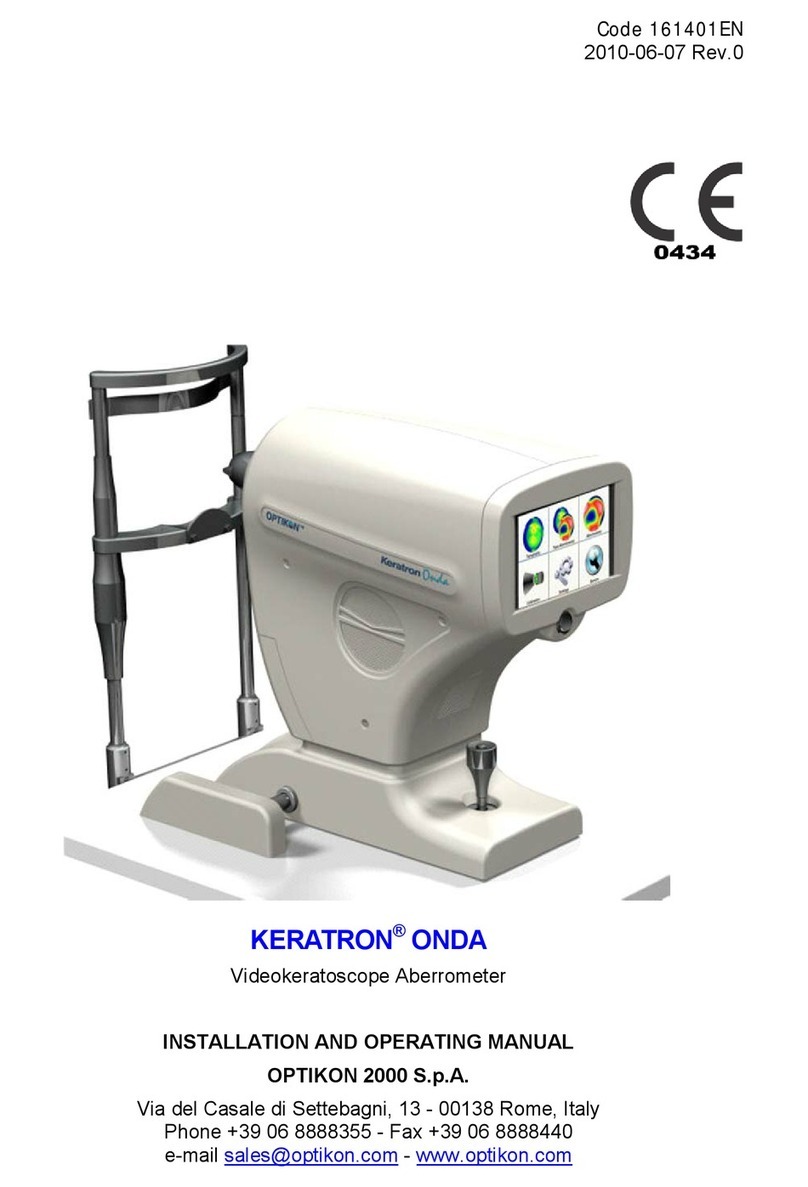
CONTENTS
1. DISCLAIMER........................................................................1-1
2. LIMITED WARRANTY CONDITIONS .............................................2-1
3. WARNINGS .........................................................................3-1
4. SYMBOLS ...........................................................................4-1
5. GENERAL INFORMATION.........................................................5-1
5.1 THEORY OF OPERATION.................................................................5-1
5.2 DESCRIPTION OF THE SYSTEM..........................................................5-1
5.3 TECHNICAL SPECIFICATIONS............................................................5-2
5.4 EMC TABLES ..............................................................................5-3
5.4.1 ELECTROMAGNETIC EMISSIONS........................................................................... 5-3
5.4.2 ELECTROMAGNETIC IMMUNITY ........................................................................... 5-4
5.4.3 RECOMMENDED SEPARATION DISTANCES ............................................................... 5-5
5.5 WIRING DIAGRAMS.......................................................................5-6
6. INSTALLATION AND MAINTENANCE............................................6-1
6.1 INTRODUCTION...........................................................................6-1
6.2OPENING THE PACKAGE AND INITIAL INSPECTION ..................................6-1
6.3 INSTALLATION PROCEDURE.............................................................6-1
7. OPERATION OF THE APPARATUS ..............................................7-1
7.1 DESCRIPTION OF THE APPARATUS.....................................................7-1
7.1.1 DISPLAY ...................................................................................................... 7-1
7.1.2 SYSTEM STATES............................................................................................. 7-1
7.1.3 INITIAL TEST OF THE SYSTEM ............................................................................ 7-1
7.1.4 STANDARD OPERATION .................................................................................... 7-1
7.1.5 TEMPERATURE CONTROL.................................................................................. 7-1
7.1.6 APPLICATION TIME CONTROL............................................................................. 7-2
7.1.7 APPLICATION TIME AND APPLIED TEMPERATURE CONTROL......................................... 7-2
7.2 CONFIGURATION OF THE UNIT.........................................................7-3
7.3 USER INTERFACE.........................................................................7-5
7.4 SOUND MESSAGES........................................................................7-6
8. USER INTERFACE..................................................................8-1
8.1 LANGUAGE SELECTION..................................................................8-1
8.2 STANDARD OPERATION..................................................................8-1
8.3 APPLICATION TIME CONTROL ..........................................................8-2
8.4 APPLICATION TEMPERATURE CONTROL...............................................8-3
8.5 SYSTEM TEST .............................................................................8-5
8.6 SHUTDOWN PROCEDURE................................................................8-6
9. CLEANING, STERILIZATION AND MAINTANENCE.............................9-1
9.1 CLEANING.................................................................................9-1
9.1.1 UNIT .......................................................................................................... 9-1
9.1.2 ACCESSORIES................................................................................................ 9-1
9.2 STERILIZATION ...........................................................................9-2
9.2.1 UNIT .......................................................................................................... 9-2
9.2.2 CRYOSURGICAL PROBES................................................................................... 9-2
9.3 MAINTENANCE............................................................................9-2
9.3.1 UNIT .......................................................................................................... 9-2
9.3.2 ACCESSORIES................................................................................................ 9-2
10. TROUBLESHOOTING GUIDE ................................................... 10-1
10.1 WARNINGS AND ERROR MESSAGES................................................... 10-1
10.2 MISCELLANEOUS PROBLEMS .......................................................... 10-2
11. ACCESSORIES ....................................................................11-1