Allergan TrueTear User manual

Patient Guide for
the TrueTear®Intranasal
Tear Neurostimulator
®

NO OTHER WARRANTY
Unless modied in writing and signed by both parties, this warranty is understood to
be the complete and exclusive agreement between the parties, superseding all prior
agreements, oral or written, and all other communications between the parties relating
to the subject matter of this agreement. No employee of Allergan or any other party is
authorized to make any warranty in addition to those made in this warranty.
Contact Information
If you wish to report a problem, please contact the provider who provided you with
the TrueTear® device, or contact Allergan:
Allergan, plc.
4410 Rosewood Drive
Pleasanton, CA 94588 USA
1-866-502-TEAR (8327)
TrueTear.com
If your dry eye symptoms become intolerable or you experience any complications
using the TrueTear® device, please contact your provider.
© 2019 Allergan. All rights reserved. All trademarks are the property of their respective
owners. Allergan.com C-0530 Revision A (Sep 2019)
®

Patient Guide for
the TrueTear®Intranasal
Tear Neurostimulator
Please read this entire guide. If you have any questions,
discuss with your provider to make sure you understand
how to use the TrueTear® Intranasal Tear Neurostimulator.
The TrueTear® Intranasal Tear Neurostimulator
(TrueTear® device) provides a temporary increase
in tear production during neurostimulation to
improve dry eye symptoms in adult patients with
severe dry eye symptoms.
Rx Only—Federal law restricts this device to sale by or on
the order of a physician or properly licensed practitioner.
Proper patient training on use of the device is required
before home use.

1
Glossary................................................................................................................2
Introduction .........................................................................................................4
Facts About Dry Eye Symptoms ..................................................................4
Indications for Use ........................................................................................4
Potential Benets of the TrueTear® Device .................................................4
Potential Complications With Using the TrueTear® Device .......................4
Contraindications, Warnings, and Precautions ..........................................5
Contraindications ...................................................................................5
Warnings .................................................................................................5
Precautions .............................................................................................7
Are You a Good Candidate for Use of the TrueTear®
Intranasal Tear Neurostimulator? ................................................................8
Questions to Ask Your Provider ...................................................................8
Summary of Important Information ............................................................9
Instructions for Use ...........................................................................................10
Overview of the TrueTear® Device Components .....................................10
Charging the Battery...................................................................................11
Assembly ......................................................................................................12
Stimulation ...................................................................................................14
Recommended Stimulation Schedule ......................................................17
Caring for Your TrueTear® Device ...............................................................17
Disposal and Replacement ........................................................................18
Bluetooth® .....................................................................................................19
FCC Compliance .........................................................................................19
Electrical Specications ..............................................................................20
Electromagnetic Compatibility ..................................................................20
Environmental Operating Conditions ......................................................21
Symbols and Markings ...............................................................................21
Summary of Clinical Studies ............................................................................22
Clinical Study OCUN-009—Single Study Visit (one-time use) ................22
Clinical Study OCUN-010—6-Month Study ..............................................23
Warranty Information........................................................................................26
Exclusions .....................................................................................................26
Warranty Claim Procedure .........................................................................27
Miscellaneous ..............................................................................................27
No Other Warranty ......................................................................................28
Contact Information ..........................................................................................28
The TrueTear®Intranasal Tear
Neurostimulator Patient Guide
Table of Contents
Section 1: Patient Guide
Section 2: Quick Start Instructions

2
Glossary
Adverse event An undesirable effect associated with use of a medical product.
Base unit The base unit produces the neurostimulation and provides a connection to the charger.
Cardiac demand pacemaker or debrillator Device (cardiac demand pacemaker) placed in or in close proximity to (debrillator) the heart to
maintain cardiac rhythm.
Clinical studies Clinical studies are conducted to evaluate the use of a drug or device.
Contraindications Cases where the TrueTear® device should not be used.
Cornea Clear tissue located in the front of the eye covering the colored area of the eye.
Disposable tip The disposable tip of the TrueTear® device connects to the base unit and is inserted into the nose.
Dry Eye symptoms
Dry eye symptoms may include, but are not necessarily limited to, sensitivity to light, grittiness, pain or
soreness, blurred vision, and poor vision. Dry eye symptoms may be caused by advanced age, contact
lens wear, certain medications, eye diseases, other medical conditions, or environmental factors.
Eligibility criteria Characteristics or criteria used to determine whether a person can participate in a clinical study.
Hypersensitivity Allergy or reaction to materials that may come into contact with the skin or to medications taken.
Intranasal Tear Neurostimulator (TrueTear® device) A device that provides small electrical pulses to stimulate tear production.
Neurostimulation Delivery of small electrical currents to activate the nerves in the nose.
Precautions A precaution provides information regarding any special care to be exercised by the provider and/or
the patient for the safe and effective use of the device.
Schirmer test A test in which a paper strip inserted inside the eyelid for several minutes to evaluate tear production.
Temporary electrical discomfort Temporary (short-term) discomfort resulting from electrical stimulation.
Warnings A warning alerts the user about serious adverse reactions and potential safety hazards, limitations in
use imposed by them, and steps that should be taken if they occur.

3
Adverse event An undesirable effect associated with use of a medical product.
Base unit The base unit produces the neurostimulation and provides a connection to the charger.
Cardiac demand pacemaker or debrillator Device (cardiac demand pacemaker) placed in or in close proximity to (debrillator) the heart to
maintain cardiac rhythm.
Clinical studies Clinical studies are conducted to evaluate the use of a drug or device.
Contraindications Cases where the TrueTear® device should not be used.
Cornea Clear tissue located in the front of the eye covering the colored area of the eye.
Disposable tip The disposable tip of the TrueTear® device connects to the base unit and is inserted into the nose.
Dry Eye symptoms
Dry eye symptoms may include, but are not necessarily limited to, sensitivity to light, grittiness, pain or
soreness, blurred vision, and poor vision. Dry eye symptoms may be caused by advanced age, contact
lens wear, certain medications, eye diseases, other medical conditions, or environmental factors.
Eligibility criteria Characteristics or criteria used to determine whether a person can participate in a clinical study.
Hypersensitivity Allergy or reaction to materials that may come into contact with the skin or to medications taken.
Intranasal Tear Neurostimulator (TrueTear® device) A device that provides small electrical pulses to stimulate tear production.
Neurostimulation Delivery of small electrical currents to activate the nerves in the nose.
Precautions A precaution provides information regarding any special care to be exercised by the provider and/or
the patient for the safe and effective use of the device.
Schirmer test A test in which a paper strip inserted inside the eyelid for several minutes to evaluate tear production.
Temporary electrical discomfort Temporary (short-term) discomfort resulting from electrical stimulation.
Warnings A warning alerts the user about serious adverse reactions and potential safety hazards, limitations in
use imposed by them, and steps that should be taken if they occur.

4
Introduction
This guide is intended to help you decide whether to use and how to use the TrueTear®
device to provide a temporary increase in tear production and improvement in dry eye
symptoms. This device provides small electrical pulses to stimulate production of your
own natural tears. The electrical pulses are delivered by a disposable tip attached to the
TrueTear® device that you will place in your nose for short periods of time.
Your provider has determined that the TrueTear® device may work for you. Please read
this entire guide and discuss your questions with your provider. You can then consider
the expected benets versus the risks and make an informed decision.
Facts About Dry Eye Symptoms
Dry eye symptoms may include, but are not necessarily limited to, sensitivity to light,
grittiness, pain or soreness, blurred vision, and poor vision. Dry eye symptoms may be
caused by advanced age, contact lens wear, certain medications, eye diseases, other
medical conditions, or environmental factors. In some people, dry eye symptoms may
be improved by increasing the amount of tears produced.
Indications for Use
The TrueTear® Intranasal Tear Neurostimulator provides a temporary increase in
tear production during neurostimulation to improve dry eye symptoms in adult
patients with severe dry eye symptoms.
Potential Benets of the TrueTear®Device
Use of the TrueTear® device will temporarily increase your tear production and
improve your dry eye symptoms, though not all patients may respond to this
device to the same degree.
Potential Complications With Using the TrueTear®Device
Potential complications include the following:
• Nasal pain, discomfort, or burning sensation
• Short-term electrical discomfort
• Nosebleeds
• Trace blood in nostril
• Nose stufness (nasal congestion)
• Excessive sneezing
• Irritation or numbness of the nose

5
• Infection, scrape (abrasion), sore formation (ulceration) or inammation
inside the nose
• Irritation or sensitivity inside the nose
• Lightheadedness
• Headaches
• Sinus pain
• Sore eye
• Facial pain or pain around the eye
• Increased saliva production
• Sensation of teeth vibrating
• Excessive runny nose
• Temporary increase in symptoms associated with nasal allergies
• Allergic reaction to contact materials
• Potential permanent scarring of the inside of nose with prolonged use
Contraindications, Warnings, and Precautions
CONTRAINDICATIONS
Contraindications are situations where it is advisable not to use the TrueTear® device.
If you have any of the following, you should NOT use the TrueTear® device:
• A cardiac demand pacemaker, implanted or wearable debrillator, or
other implanted metallic or electronic device (eg, cochlear implant)
in the head or neck
• Chronic or recurrent nosebleeds, a bleeding disorder (eg, hemophilia), or
another condition that can lead to increased bleeding
• A known hypersensitivity (allergy) to the stainless steel material that comes
into contact with the inside of your nose
WARNINGS
Warnings alert the user about serious adverse reactions and potential safety hazards,
limitations in use imposed by them, and steps that should be taken if they occur, as
identied below:
• Follow the Instructions for Use when using the TrueTear® device.
• Do not use the TrueTear® device if electronic monitoring equipment is being
used. This type of equipment includes heart monitors or electrocardiogram
(ECG) alarms since this equipment may not operate properly when the
TrueTear® device is being used.

6
Contraindications, Warnings, and Precautions
WARNINGS (continued)
• Do not use the TrueTear® device when in the bath or shower.
• Do not use the TrueTear® device while driving, operating machinery, or during
any activity in which sneezing or watery eyes may put you at risk of injury.
• Do not apply the TrueTear® device to the neck, chest, or areas other than the nose.
• Do not continue using the TrueTear® device if your nose is irritated since further
use may cause injury to the tissues inside your nose.
• Do not use the TrueTear® device within 3 feet of shortwave or microwave
therapy equipment since this equipment may make the stimulation from
the TrueTear® device unstable.
• Do not use the TrueTear® device in the presence of a ammable anesthetic
mixture with air or with oxygen or nitrous oxide as there is a remote possibility
(comparable to the risk of a mobile phone) it could ignite the gas.
• Use only manufacturer's supplied accessories.
•The TrueTear® device is limited only to the improvement in dry eye
symptoms as the safety and effectiveness in the treatment of dry eye
disease has not been established.
•In a clinical study, the safety and effectiveness of the TrueTear® device
was evaluated over a 6-month period of time. The safety and effectiveness
of the TrueTear® device for longer periods of use have not been established.
Your provider may periodically check your nose if the TrueTear® device is
used over a longer period of time.
•The clinical study was not designed to evaluate any changes in
nerve sensitivity.
•The safety of the TrueTear® device has not been established in the following
conditions and patient populations:
• Pregnancy
• Patients under 22 years of age
• Nasal (nose) or sinus surgery, including a history of nasal cautery, or
signicant trauma
• Severe nasal airway obstruction (such as severe septal deviation or inferior
turbinate hypertrophy) or vascularized polyp (abnormal nasal mucosa with dense
network of blood vessels)

7
WARNINGS (continued)
• Disabling arthritis, neuropathy, severe dexterity impairment or limited
motor coordination that would affect your ability to use or handle the
TrueTear® device
• Active and severe:
• Systemic allergy
• Chronic seasonal allergies
• Rhinitis or sinusitis requiring treatment such as
antihistamines, decongestants, oral or aerosol steroids
• Untreated nasal infection
PRECAUTIONS
Precautions provide information regarding any special care to be exercised by the
provider and/or patient for the safe and effective use of the TrueTear® device.
• Consult your provider before using the TrueTear® device.
• If you feel pain, discomfort, or numbness in your nose with
higher levels of stimulation or a longer duration of stimulation, reduce the level
and/or the number of times you use the TrueTear® device. If symptoms persist,
discontinue use and contact your provider.
• Discard the disposable tip every 28 days and replace with a new tip for proper
operation and good hygiene.
• Remove any studs, nose rings, or other piercings from the nose prior to using
the TrueTear® device as this could obstruct the device and/or cause discomfort if
the electrical stimulation is conducted to surrounding areas.
• Do not use prescription eye medications (eye drops, gels, or ointments) or nasal
sprays within 30 minutes before or after using the TrueTear® device.
• Consult your provider before use if you have suspected or diagnosed
heart disease.
• The TrueTear® device should be kept out of the reach of children.
• If you have a severe fear of placing anything in your nose, you may not
be able to use the TrueTear® device.
• Follow the cleaning and caring instructions provided.
• Failure to replace the tip as directed will prevent the device from
providing stimulation.

8
Are You a Good Candidate for Use of the TrueTear®
Intranasal Tear Neurostimulator?
You are a good candidate for the TrueTear® device if you:
• Are at least 22 years old.
• Have dry eye symptoms.
• Are able to use the TrueTear® Intranasal Tear Neurostimulator.
• Do not have a cardiac demand pacemaker, implanted or wearable debrillator,
or other implanted metallic or electronic device in the head or neck.
• Do not have a known hypersensitivity to the any of the device materials that
contact you.
• Do not have chronic or recurrent nosebleeds, a bleeding disorder or another
condition that can lead to increased bleeding.
Questions to Ask Your Provider
You may want to ask your provider the questions below to help you decide if the
TrueTear® device is right for you.
• What other options do I have for my dry eye symptoms?
• What are the benets of the TrueTear® device?
• Can I use the TrueTear® device as often as I want?
• Will I be able to use articial tears, gels and ointments in addition to using the
TrueTear® device?
• Will I be able to use dry eye drugs in addition to using the TrueTear® device? Are
there any risks if I use the TrueTear® device with dry eye drugs?

9
Summary of Important Information
• The TrueTear® device provides a temporary increase in tear production during
use resulting in an improvement in dry eye symptoms in adult patients with
severe dry eye symptoms.
• You should not use the TrueTear® device if you have any of the following
conditions:
• A cardiac demand pacemaker, implanted or wearable debrillator, or
other implanted metallic or electronic device in the head or neck
• Chronic or recurrent nosebleeds, a bleeding
disorder or another condition that can lead to increased bleeding
• A known hypersensitivity (allergy) to the stainless steel material that
comes into contact with the inside of your nose
• You should follow all instructions to make sure you use the TrueTear®
device correctly.
• Please call 1-800-433-8871 to report an adverse event.
10
Instructions for Use
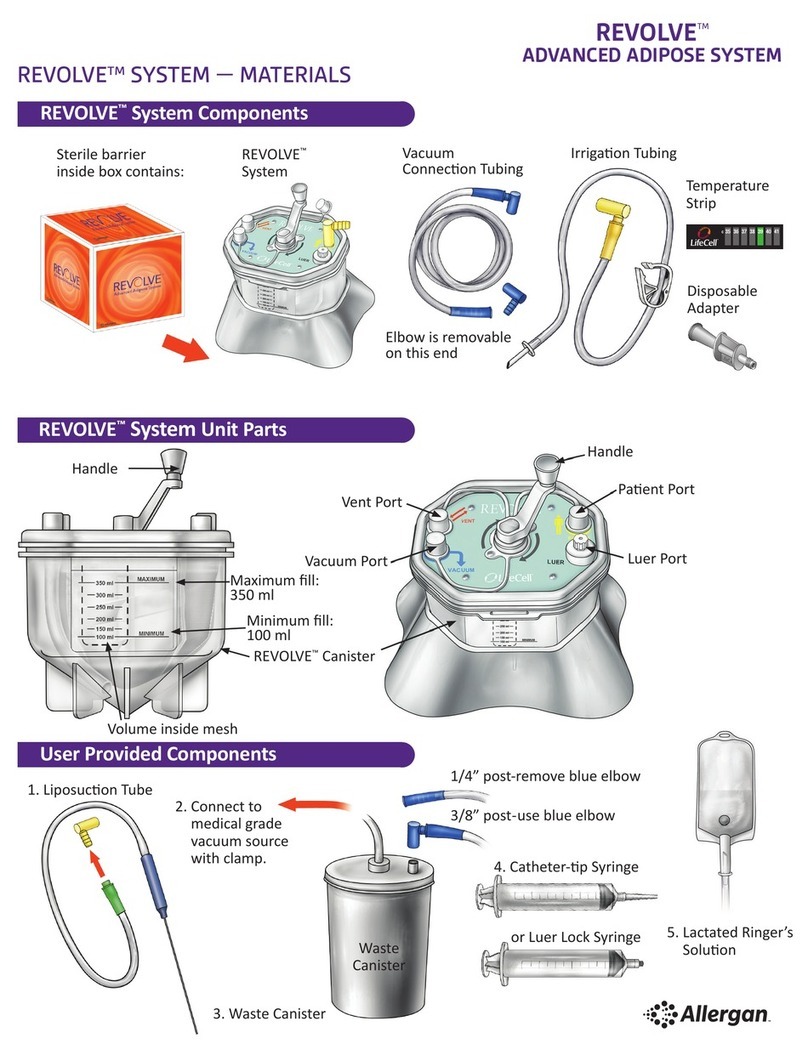
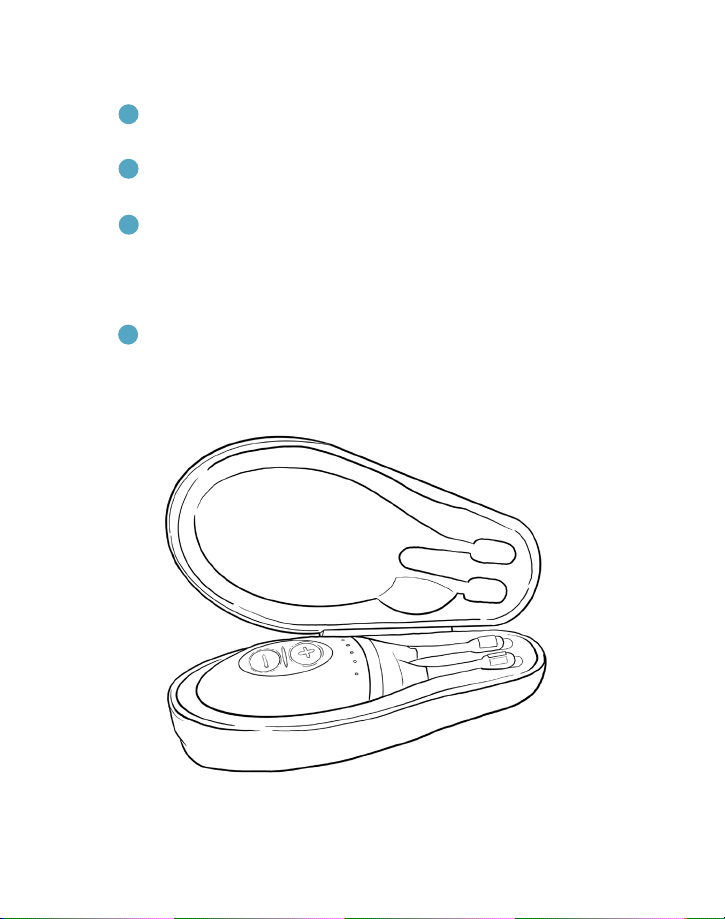
Figure 1. TrueTear®components.
BaseDisposable Tip Case
OVERVIEW OF THE TRUETEAR®DEVICE COMPONENTS
The TrueTear® device consists of three parts.
1 A disposable tip, which is inserted into the nasal cavity
and provides the contact surface for the stimulation
in the nose
2 A base, which produces the stimulation
3 A case that protects and charges the device
in between uses
The disposable tip (tip) is connected to the base for
stimulation. The tip provides the contact for conducting
the stimulation current, which is produced by the base.
All images shown in this guide are for referencing only.

11
USING AND CHARGING THE DEVICE
NOTE: Only use the provided AC adapter.
1
Open the case and place the base with the attached tip inside
the case. the base (front) should face up.
2 Close the case. If the case is not closed, the base may not
charge properly.
3 Connect the provided cable and adapter to the case and plug
the adapter into an active (120-240V) outlet.
4 The case bottom will glow orange when the device is charging
and glow blue when the device is fully charged.
Figure 2. Charging the TrueTear®device.
Orange glow indicates device is
charging
Blue glow
indicates device is fully charged
Charge the base
before rst use.
A fully charged
device should last
several days.
12
STIMULATION INSTRUCTIONS
1 Remove a new disposable tip from the pouch.
Figure 3.
13
STIMULATION INSTRUCTIONS
1 Remove a new disposable tip from the pouch.
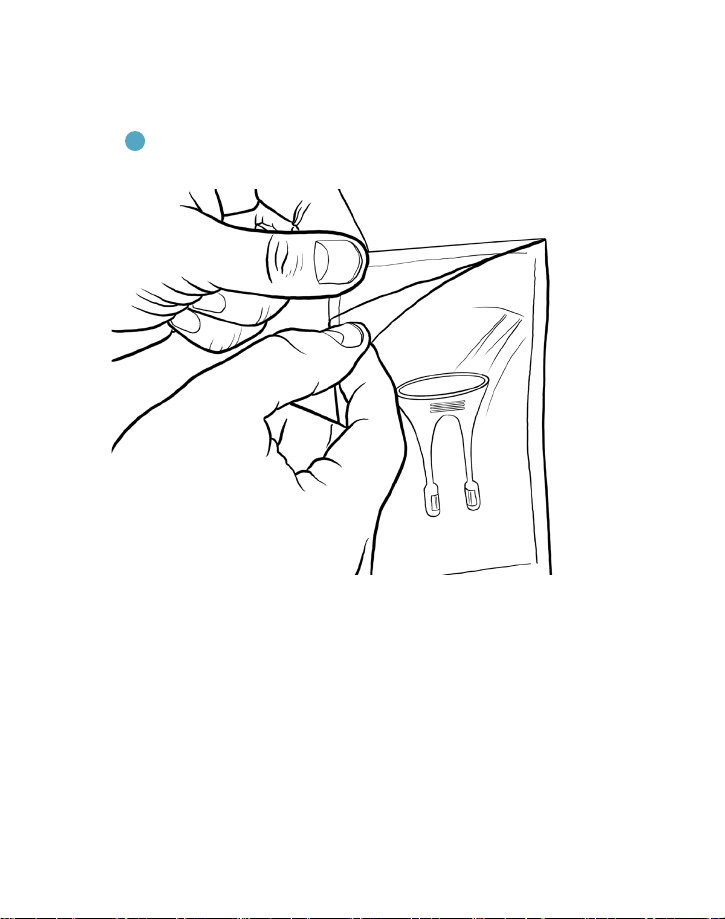
Figure 4. Align the tab to the notch for setup. The tip only ts one way.
3 Connect the tip to the base by aligning the post on the underside of
the tip with notch on base, then rotate forward until the tip snaps into
place, as shown in Figure 4.

14
There are 5 stimulation intensity levels. The base vibrates briey when the + or - button
is pressed to indicate an increase or decrease in stimulation level. The blue lights will be
lit to indicate the stimulation level selected.
Your provider will conrm that you understand these instructions, including having you
demonstrate the stimulation technique and the tearing response, prior to prescribing
the TrueTear® device and, if necessary, at subsequent visits:
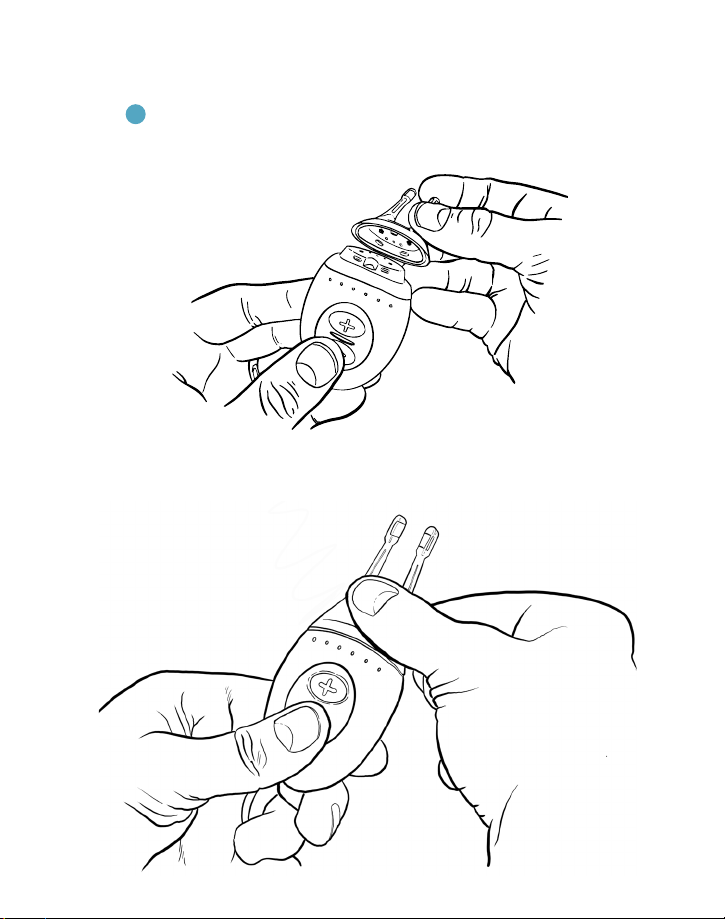
1 With the TrueTear® device fully assembled, hold the + button for 2 seconds to turn
on the device. A steady white light will apear on the base indicating that the device
is on. as shown in Figure 5.
2.Press the + button to select a desired stimulation intensity level. Blue lights show
the level selected. Always start on level 1.
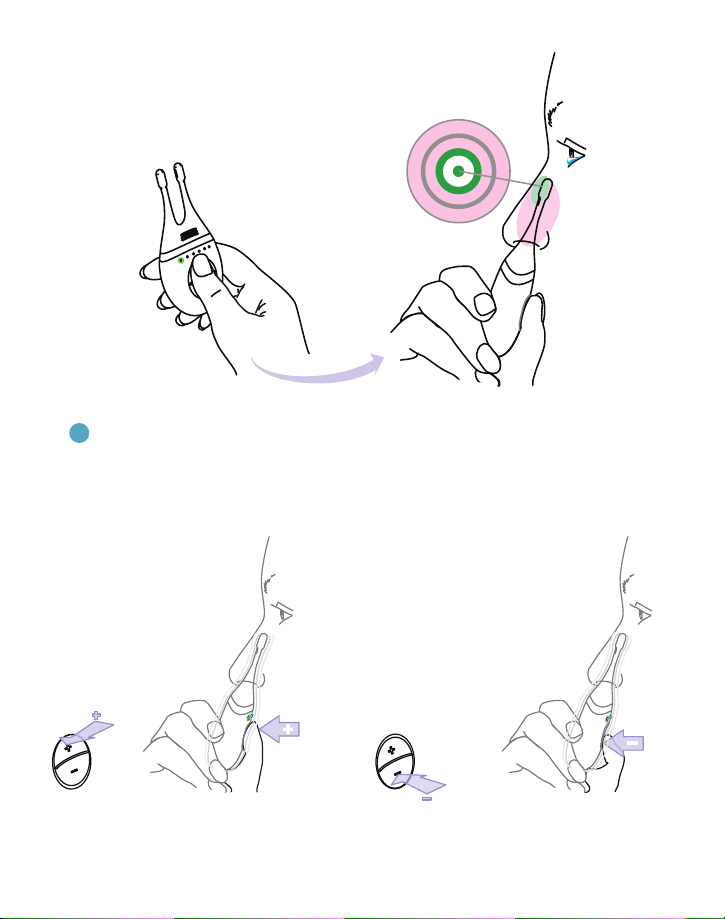
3.Place thumb near buttons of base and gently insert the tip into the nose with the
back of the base facing out, as shown in Figure 5.
4. For effective stimulation, insert the tip toward the top and front of the nose, as
shown in Figure 6.
Figure 5. Turning on the TrueTear®device and placing it into the nose.
Figure 7. Adjust stimulation by pressing the + or – buttons.
15
Figure 5. Turning on the TrueTear®device and placing it into the nose.
Figure 6. Target zone for correct insertion of disposable tip.
5.The + button is for increasing the intensity and the – button is for decreasing the intensity.
You may gradually increase and adjust intensity (using the + and – buttons) until you feel a
gentle tingling in your nose; this feeling lets you know that you are stimulating the correct
tissue location and tears will form.
Insert tip into your nose,
as far as is comfortable.
Figure 7. Adjust stimulation by pressing the + or – buttons.
Rest thumb on the + or — button.
Press + or — to change levels
if desired.
Always start on level 1.
16
Figure 8. Cover attached to base unit to protect disposable tip.
6 You may reposition the tip inside the nose for desired stimulation.
The feeling should be mild at its maximum intensity.
7 Remove the tip from your nose at any time if you feel uncomfortable
during stimulation.
The device turns off automatically after three (3) minutes. You can also turn
it off manually by pressing the - button for 2 seconds. The device will vibrate
and the lights will turn off to indicate the device is off.
Note: You can turn the device off as soon as tears start forming.
When nished, clean the TrueTear® device with an alcohol wipe, if needed
(see CARING FOR YOUR DEVICE), and store the device in the case provided.
8
9

17
RECOMMENDED STIMULATION SCHEDULE
Use the TrueTear® device at least twice a day, as needed. Stimulation longer than
3 minutes is not recommended, and you should wait for at least 60 minutes before
proceeding to the next application. The device has a built-in single-day usage limit
of 30 minutes. If this daily limit has been reached, the TrueTear® device will turn on
and then off immediately. The device will not deliver stimulation.
Replace the tip every 28 days with a new tip. When a tip has 7 days of usage left before
expiration, the device will vibrate 3 times when the device is turned on or off. If the device
is turned on without a tip or with an expired tip, the device will vibrate 3 times but will not
deliver stimulation.
WHAT YOU'LL SEE WHAT IT MEANS
A single, steady white light when device
is turned on.
Device is on. No stimulation is delivered.
A single, ashing white light when the
device is turned on.
Battery is running low. Place base in case.
All lights ash on and off. Daily stimulation limit of 30 minutes has
been reached.
Figure 9. Cleaning with alcohol wipes.
CARING FOR YOUR TRUETEAR®DEVICE
1 Use alcohol wipes to clean the device and store
the device in the case between uses.
2 Use alcohol wipes to clean tthe case as needed.
Other manuals for TrueTear
2
Table of contents
Other Allergan Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual