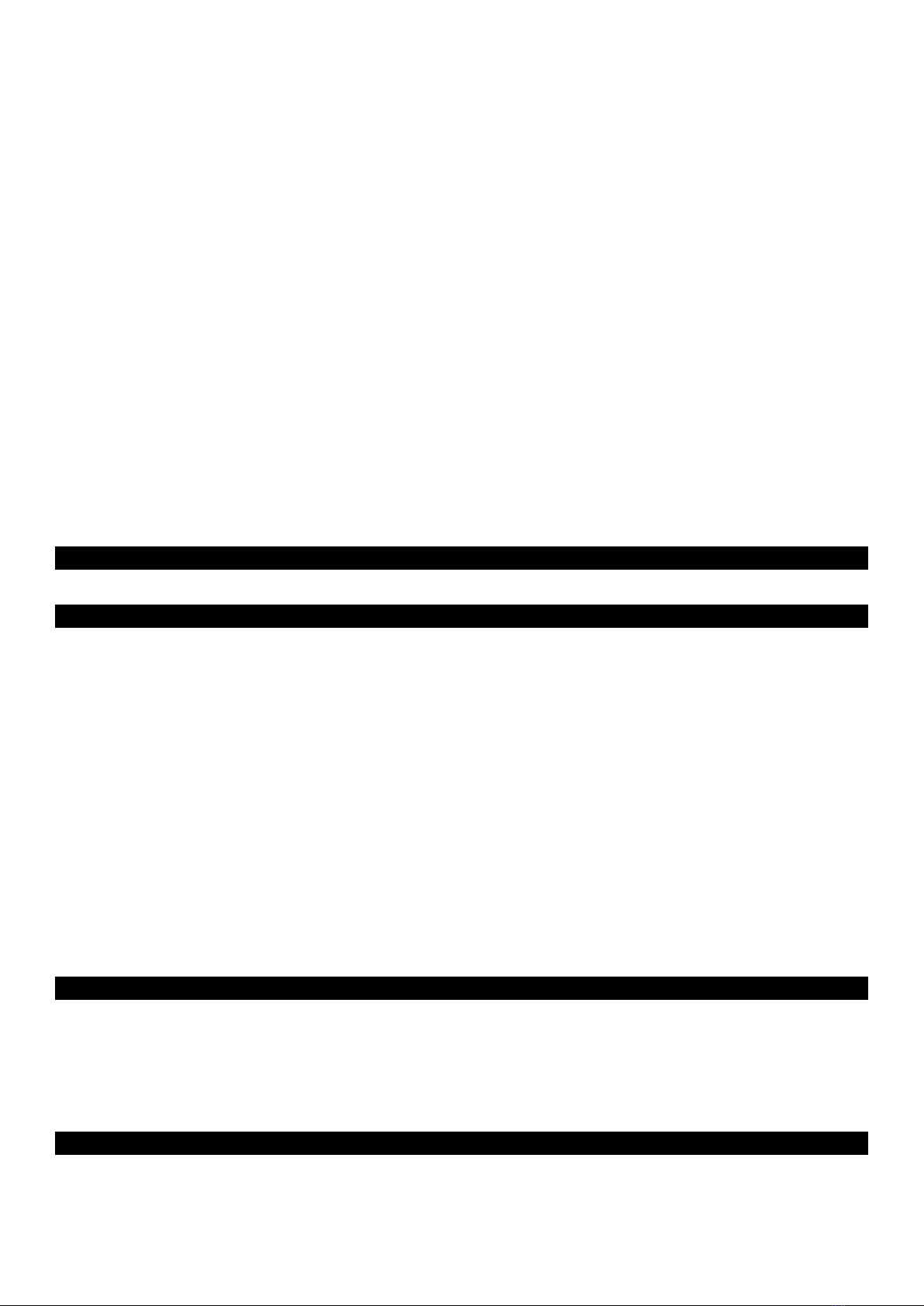D.SPEC.05.14 Pulse oxymètre MD300-C15D - Mode d'emploi_EN
v. 28/06/2019 4
Fingertip
Pulse Oximeter
USER MANUAL
Ver3.0C1/C4
General Description
Oxygen Saturation is a percentage of Oxyhemoglobin (HbO2) capacity, compounded with oxygen,
by all combinative hemoglobin (Hb) capacity in blood. In other words, it is consistency of
Oxyhemoglobin in blood. It is a very important parameter for the Respiratory Circulation System.
Many respiratory diseases can result in oxygen saturation being lowered in human blood.
Additionally, the following factors can reduce oxygen saturation: Automatic regulation of organ
dysfunction caused by Anesthesia, Intensive Postoperative Trauma, injuries caused by some
medical examinations. That situation might result in light-headedness, asthenia, and vomiting.
Therefore, it is very important to know the oxygen saturation of a patient so that doctors can find
problems in a timely manner.
The fingertip pulse oximeter features low power consumption, convenient operation and portability.
Place one fingertip into the photoelectric sensor for diagnosis and the pulse rate and oxygen
saturation will appear on the display. It has been proven in clinical experiments that it also features
high precision and repeatability.
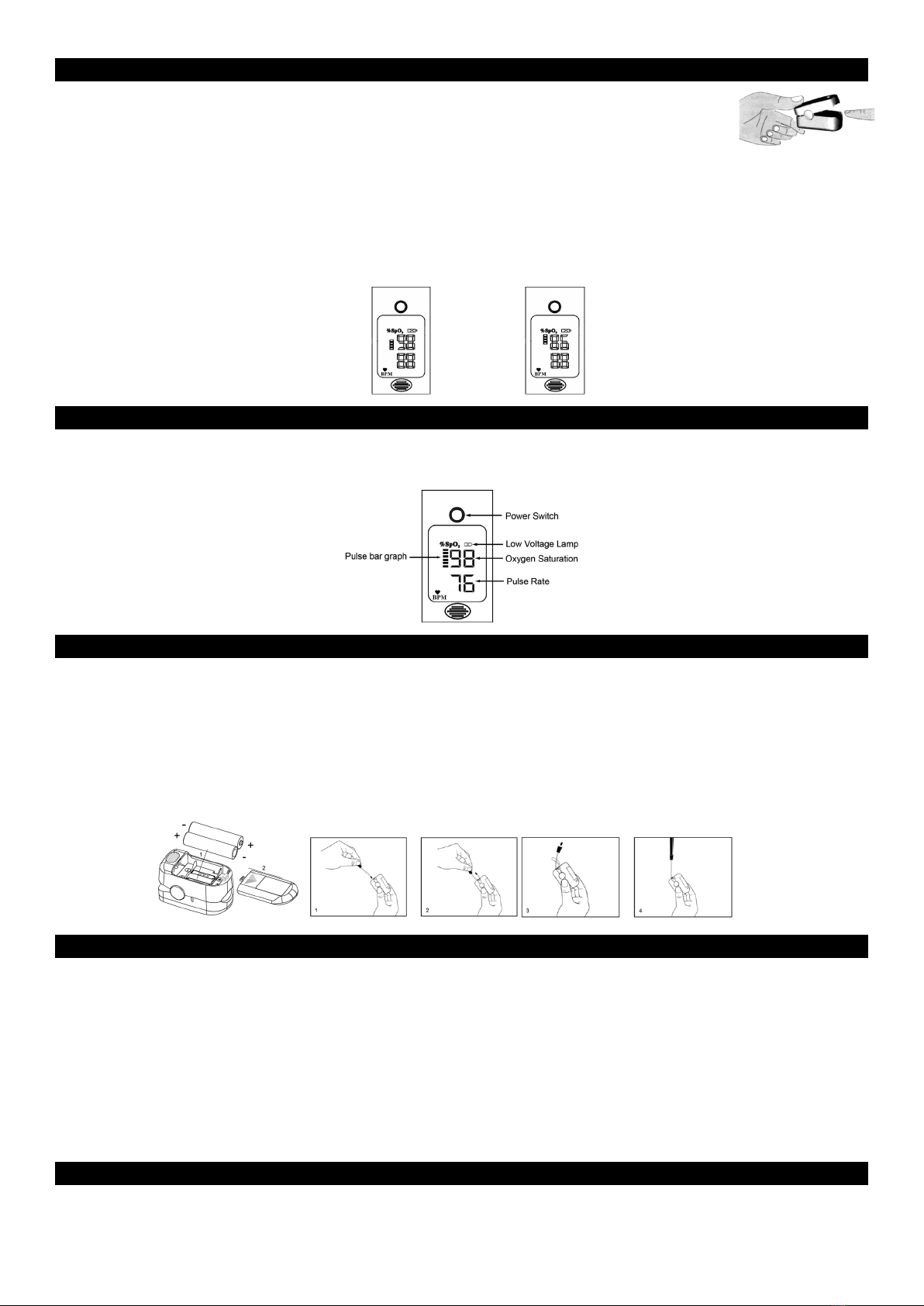
Measurement Principle
Principle of the oximeter is as follows: A mathematical formula is established making use of Lambert
Beer Law according to Spectrum Absorption Characteristics of Reductive hemoglobin (RHb) and
Oxyhemoglobin (HbO2) in red and near-infrared zones. Operation principle of the instrument:
Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse
Scanning and Recording Technology, so that two beams of different wavelength of lights (660nm
red and 905nm near infrared light) can be focused onto a human nail tip through a clamping
finger-type sensor. A measured signal obtained by a photosensitive element, will be shown on the
oximeter’s display through process in electronic circuits and microprocessor.
Diagram of Operation Principle
1. Red and Infrared-ray Emission Tube
2. Red and Infrared-ray Receipt Tube
Precautions For Use
1. Before use, carefully read the manual.
2. Operation of the fingertip pulse oximeter may be affected by the use of an electrosurgical unit
(ESU).
3. The fingertip pulse oximeter must be able to measure the pulse properly to obtain an accurate
SpO2measurement. Verify that nothing is hindering the pulse measurement before relying on
the SpO2measurement.
4. Do not use the fingertip pulse oximeter in an MRI or CT environment.
5. Do not use the fingertip pulse oximeter in situations where alarms are required. The device has
no alarms. It is not for continuous monitoring.
6. Do not use the fingertip pulse oximeter in an explosive atmosphere.
7. The fingertip pulse oximeter is intended only as an adjunct in patient assessment. It must be
used in conjunction with other methods of assessing clinical signs and symptoms.
8. In order to ensure correct sensor alignment and skin integrity, the maximum application time at
a single site for our device should be less than half an hour.
9. Do not sterilize the device using autoclaving, ethylene oxide sterilizing, or immersing the device