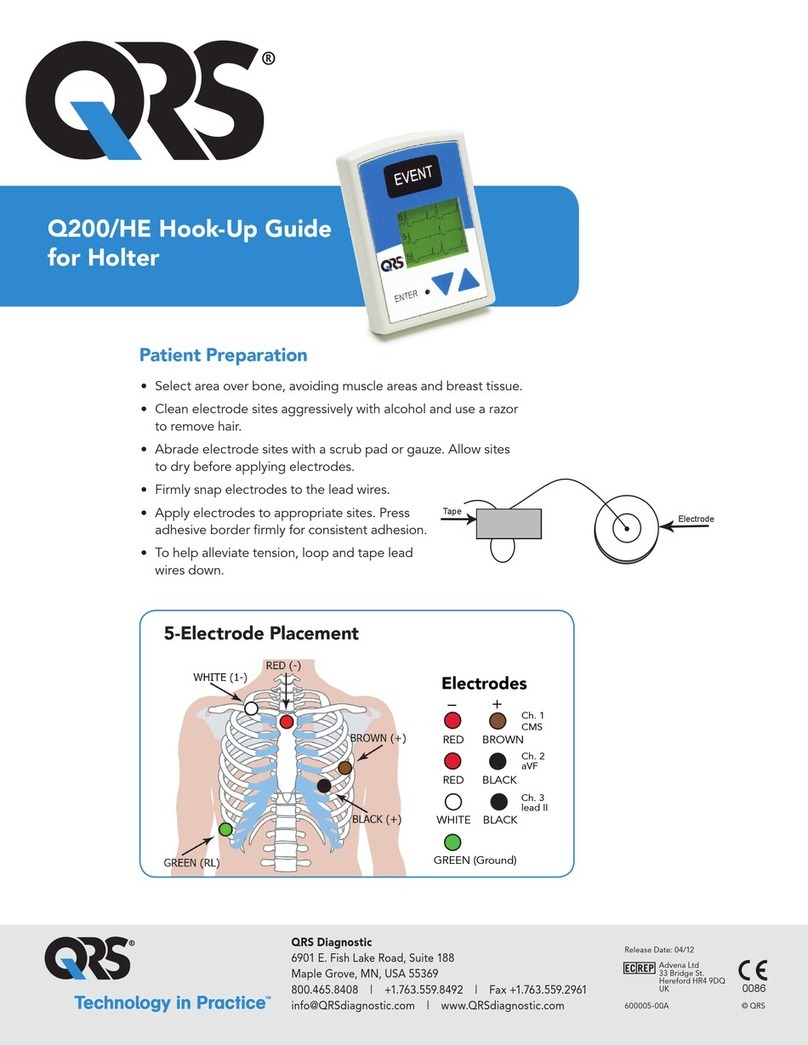
12
Floors should be wood, concrete or ceramic tile. If floors are covered with
synthetic material, the relative humidity should be at least 30%
Electrostatic Discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 air
Electromagnetic Environment Guidance
IEC 60601 Test Level
Immunity Test
Electrical fast transient burst
IEC 61000-4-4
(Note: Not applicable for Biolog & Sensaire)
Surge
IEC 61000-4-5
(Note: Not applicable for Biolog & Sensaire)
Voltage dips, short interruptions and voltage
variations on power supply input lines
IEC 61000-4-11
(Note: Not applicable for Biolog & Sensaire)
Power frequency (50/60 Hz) magnetic field
IEC 61000-4-8
Note UT is the a.c. mains voltage prior to application of the test level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
±2 kV for power supply lines
±1 kV for input/output lines
±1 kV differential mode
±2 kV common mode
<5% UT
(>95% dip in UT) for 0.5 cycle
40% UT
(60% dip in UT) for 5 cycles
70% UT
(30% dip in UT) for 25 cycles
<5% UT
(>95% dip in UT) for 5 sec
3 A/m
3 Vrms
150 KHz to 80 Mz
3 V/m
80 MHz to 2.5 GHz
<5% UT
(>95% dip in UT) for 0.5 cycle
40% UT
(60% dip in UT) for 5 cycles
70% UT
(30% dip in UT) for 25 cycles
<5% UT
(>95% dip in UT) for 5 sec
±2 kV for power supply lines
±1 kV for input/output lines
±1 kV differential mode
±2 kV common mode
±6 kV contact
±8 air
Compliance Level
3 A/m
3 Vrms
3 V/m
Mains power quality should be that of a typical commercial or hospital environment
Mains power quality should be that of a typical commercial or hospital environment.
If the user of QRS medical devices requires continued operation during power
mains interruptions, it is recommended that the computer to be used is powered
by an uninterruptible power supply or a battery.
Power frequency magnetic fields should be at levels characteristic of a typical loca-
tion in a typical commercial or hospital environment.
Portable and mobile RF Communications equipment should be used no closer to
any part of QRS Diagnostic medical devices, including cables, than the
recommended separation distance calculated from the equation applicable to the
frequency of the transmitter.
Recommended separation distance
Where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer and d is the recommended separation
distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic
site survey, a should be less than the compliance level in each frequency range. b
Interface may occur in the vicinity of equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which QRS medical devices
are used exceeds the applicable RF compliance level above, QRS medical devices should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as
reorienting or relocating QRS medical devices.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communications equipment
and QRS Diagnostic medical devices.
QRS Diagnostic medical devices are intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of QRS Diagnostic medical
devices can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and QRS Diagnostic medical
devices as recommended below, according to the maximum output power of the communications equipment.
Rated maximum output power of transmitter
W
Separation distance according to frequency of transmitter
150 kHz to 80 MHz 800 MHz to 2.5 GHz
0.01 0.12 0.23
0.38 0.73
2.3
1.2
1.2
7.3
23
3.8
3.8
12
0.1
1
10
100
For transmitters rated at a maximum output power not listed above, the recommended separation distance din metres (m) can be estimated using the equation applicable to the frequency of the
transmitter, where Pis the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
Mains power quality should be that of a typical commercial or hospital environment
0.12
80 MHz to 800 MHz
0.38
QRS Diagnostic medical devices are intended for use in the electromagnetic environment(s) specified below. Users of this equipment should assure that
it is used in such environment(s).
Guidance and manufacturer's declaration - electromagnetic emissions and immunity