NOTE: The procedures for testing swab specimens versus liquid specimens in viral transport media are different. Read carefully.
All specimens must be at room temperature before testing.
Expiration date: Check expiration date on each individual test package or outer box before using. Do not use any test past the expiration date on the label.
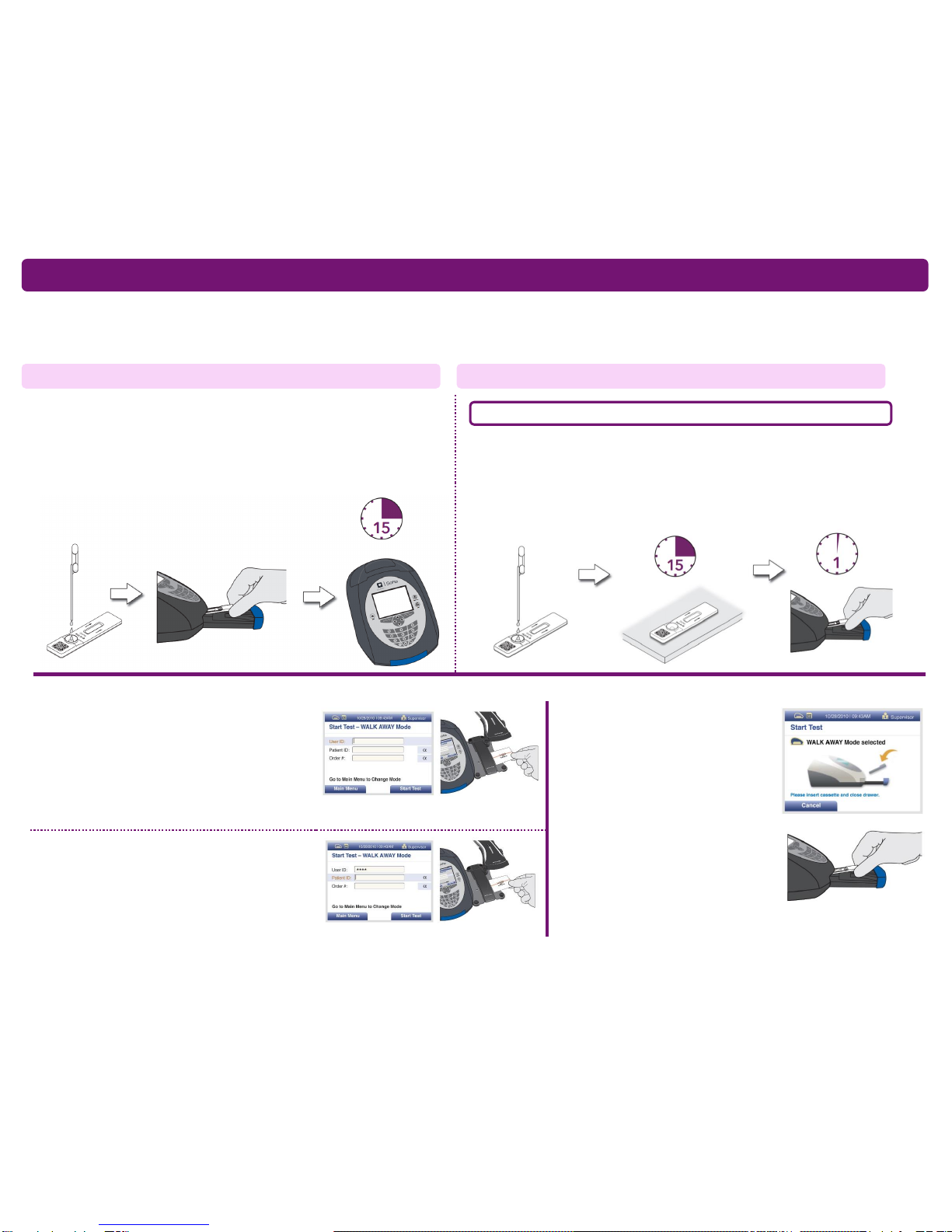
Verify that Sofia or
Sofia 2 is set to the
desired Mode:
WALK AWAY or
READ NOW. See
the “Using Sofia”or
“Using Sofia 2”
section for more
information.
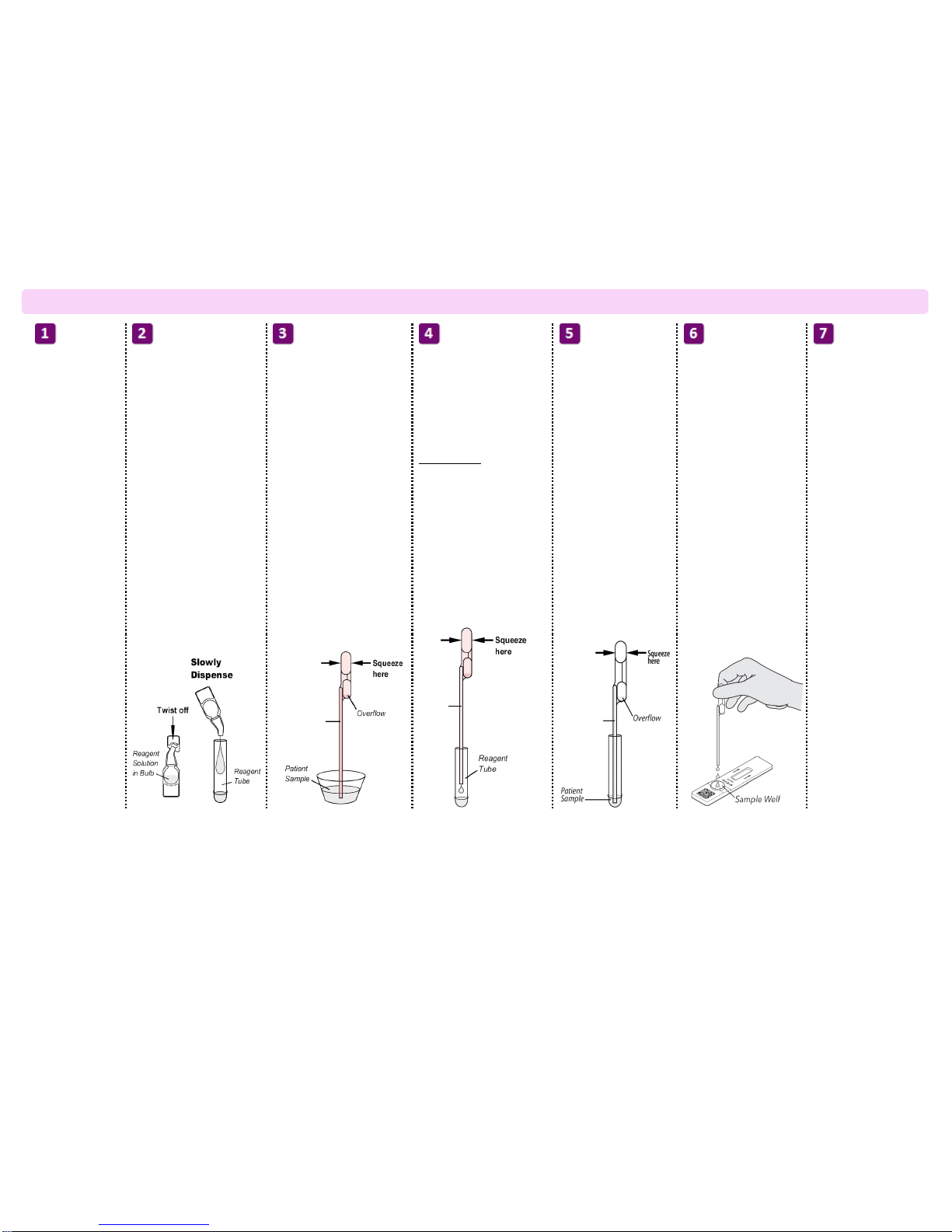
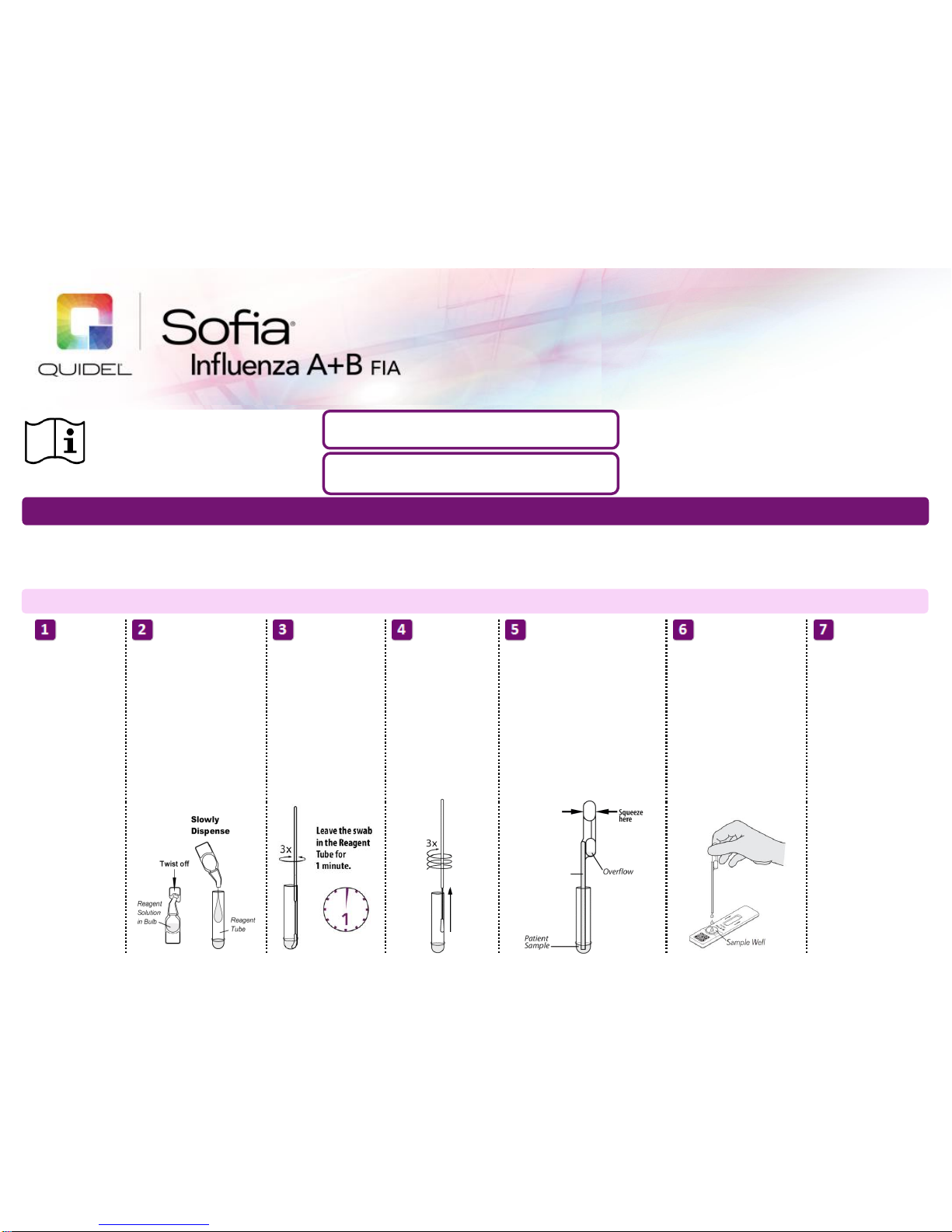
Dispense all of the Reagent
Solution into the Reagent
Tube. Swirl the Reagent Tube
to dissolve its contents.
Place the patient swab
sample into the Reagent
Tube. Roll the swab at
least 3 times while
pressing the head
against the bottom and
side of the Reagent
Tube.
Roll the Swab head
against the inside of the
Reagent Tube as you
remove it. Dispose of
the used Swab in your
biohazard waste.
Fill the provided Small, Clear
120 µL Fixed Volume Pipette with
patient sample from the Reagent
Tube.
To fill the Fixed Volume Pipette
with the patient sample:
a) FIRMLY squeeze the top bulb.
b) Still squeezing, place the Pipette
tip into the sample.
c) With the Pipette tip still in the
sample, release pressure on bulb
to fill the Pipette.
Firmly squeeze the top bulb to
empty the contents of the Small,
Clear 120 µL Fixed Volume Pipette
into the Test Cassette sample well.
Extra liquid in the overflow bulb is
OK.
NOTE: The Fixed Volume Pipette is
designed to collect and dispense the
correct amount of patient sample.
Discard the Pipette in your
biohazard waste.
NOTE: Do not pour sample from the
Reagent Tube. Use the provided
Small, Clear 120 µL Fixed Volume
Pipette.
Proceed to the
“Using Sofia”or
“Using Sofia 2”
section to complete
the test.
Swab Test Procedure (Nasal/Nasopharyngeal)
Study the Package Insert and User Manual
thoroughly before using Quick Reference
Instructions. This is not a complete
Package Insert.
IMPORTANT! Read instructions carefully before
beginning. The test procedure below is unique to the
Sofia Influenza A+B FIA and may differ from other Sofia
FIA procedures.
CLIA Complexity: Waived for direct nasal swab, nasopharyngeal
swab and nasopharyngeal aspirate/wash specimens
QUICK REFERENCE
INSTRUCTIONS
For use with Sofia and Sofia 2.
Rx only
CLIA Complexity: Moderate for nasopharyngeal swab and
nasopharyngeal aspirate/wash specimens eluted in
transport media