General information
The cobas b 101 instrument is intended for professional
use in a clinical laboratory setting or at the point of care.
The instrument can be configured according to your
institution’s needs, and features such as requiring an
operator ID may or may not be used.
For all questions about cobas b 101 instrument that are
not answered in the quick reference guide, operator’s
manual or package inserts, contact your Roche
representative.
If so configured, the cobas b 101 instrument prevents
patient testing when controls have failed (QC lockout).
QC lockout occurs when:
• Patient testing is attempted and controls have not
been run in the time interval or frequency designated
by your institution.
• Controls have been run but the control values were
outside the allowed range.
Error messages are displayed to alert you to a condition
that needs attention. Take the action suggested on the
screen, select
ll
, and proceed with testing.
1. Visit our website at www.roche.com.
2. Choose your country to find the appropriate local
office contact information.
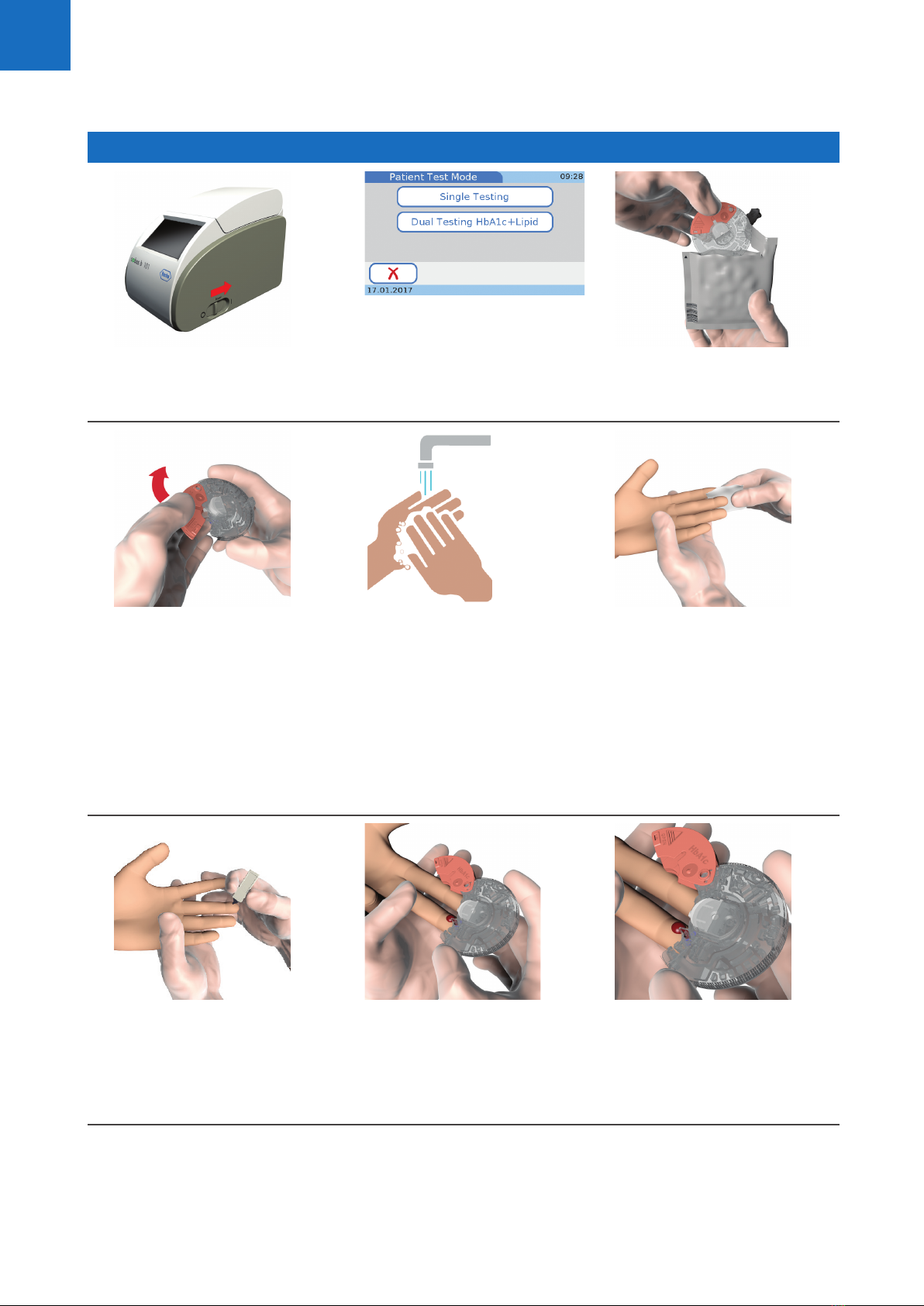
You need the following materials to perform patient and
control tests:
• cobas b 101 instrument
• cobas HbA1c Test
• cobas Lipid Panel
• cobas CRP Test
• cobas HbA1c Control
• cobas Lipid Control
• cobas CRP Control
• cobas HbA1c QC Info Disc, cobas Lipid QC Info Disc
and cobas CRP QC Info Disc (included in the
respective control kit)
To find your Roche contact details
Materials
5