6
S-19-01-1218-U0001 V 1.5
electronic equipment might interfere with the
operation of the device. We recommend that the
MS handpiece ca le kept at least 6 to 9 inches (15
to 23 cm) away from any device and their leads
during use.
‧
During using the unit, make sure that water is
flowing continuously. If the handpiece overheats
please check the water supply, and stop using the
unit for a while.
Section 7: Infection Control
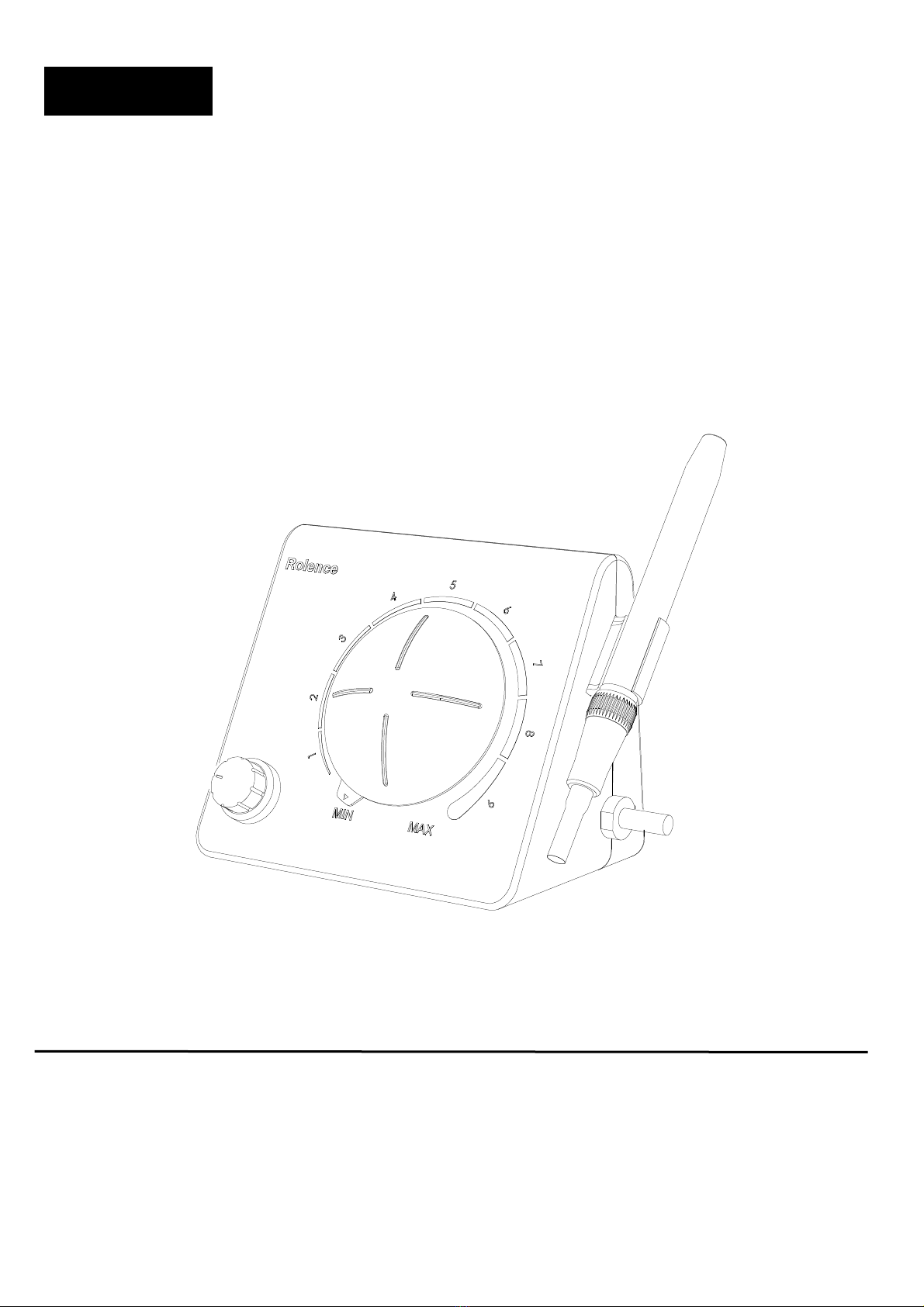
7.1 Function:
The Elitedent
®
MS-30 Ultrasonic Scaler is designed
for use in prophylaxis treatments periodontia, and
other areas of operative dentistry.
When used in prophylaxis treatment, the unit
operates with a fine water spray, requiring little of
the physical exertion necessary with hand
instruments. It easily and effectively removes
stu orn calculus and stains oth supragingivally
and su gingivally, leaving crown and root surfaces
clean and smooth.
7.2 General Infection Control
Recommendations
‧
As with all dental procedures, the use of standard
personal protection equipment (i.e., wearing a
face mask, eyewear, or face shield, gloves and
protective gown) is recommended.
‧
For maximal operator and patient safety, carefully
follow section 12 system maintenance and care
information detailed in the operating instruction.
‧
As with high speed handpieces, and other dental
devices, the com ination of water and ultrasonic
vi ration from your ELiTEDENT
®
Ultrasonic Scaler
will create aerosols. With proper technique, much
of the aerosol dispersion can e effectively
controlled and minimized. Please carefully follow
the procedural guide lines in this manual
regarding the use of your ultrasonic scaler.
‧
Always flush your ELiTEDENT
®
Ultrasonic Scaler
at least 20 seconds with highest flow after each
treatment. Refer to more information in section
12.
‧
Clean and disinfect the steriliza le sleeve of
handpiece etween patients. The steriliza le
sleeve of handpiece can e autoclaved up to 135
℃
for at least 10 mincutes.
Sterilizing:
1. Autoclave: compliant with standard EN 13060.
2. Place steriliza le sleeve of handpiece and
pouched ultrasonic insert into a steam autoclave.
After warm-up is completed, operate at a
sterilizing temperature and pressure of 273° F/31
psi (134°C/216 kPa) for 18 minutes, followed y a
20-30 minute drying time.
3. To maintain sterility, the insert should remain in
the sealed pouch until it is ready for use.
DO NOT USE Cold sterilization solution.
7.2 Water Supply Recommendations
‧
It is highly recommended that all dental water
supply systems should conform to applica le CDC
(Centers for Disease Control and Prevention) and
ADA (American Dental Association) standards,
and that all recommendations e followed in
terms of flushing, chemical flushing, and general
infection control procedures. See sections 7.2 and
12.